PROTOPAM CHLORIDE- pralidoxime chloride injection, powder, lyophilized, for solution
Protopam Chloride by
Drug Labeling and Warnings
Protopam Chloride by is a Prescription medication manufactured, distributed, or labeled by Baxter Healthcare Corporation, BAXTER PHARMACEUTICAL SOLUTIONS, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
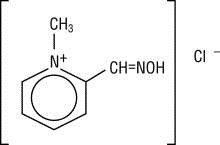
Chemical name: 2-formyl-1-methylpyridinium chloride oxime. Available in the United States as PROTOPAM Chloride for Injection (PROTOPAM Chloride), pralidoxime chloride is frequently referred to as 2-PAM Chloride.
Structural formula:
Pralidoxime chloride occurs as an odorless, white, nonhygroscopic, crystalline powder which is soluble in water. Stable in air, it melts between 215º and 225º C, with decomposition.
The specific activity of the drug resides in the 2-formyl-1-methylpyridinium ion and is independent of the particular salt employed. The chloride is preferred because of physiologic compatibility, excellent water solubility at all temperatures, and high potency per gram, due to its low molecular weight.
Pralidoxime chloride is a cholinesterase reactivator.
PROTOPAM Chloride for intravenous injection or infusion is prepared by cryo-desiccation. Each vial contains 1000 mg of sterile pralidoxime chloride, and sodium hydroxide to adjust pH, to be reconstituted with 20 mL of Sterile Water for Injection, USP. The pH of the reconstituted solution is 3.5 to 4.5. Intramuscular or subcutaneous injection may be used when intravenous injection is not feasible.
-
CLINICAL PHARMACOLOGY
The principal action of pralidoxime chloride is to reactivate cholinesterase (mainly outside of the central nervous system) which has been inactivated by phosphorylation due to an organophosphate pesticide or related compound. The destruction of accumulated acetylcholine can then proceed, and neuromuscular junctions will again function normally. Pralidoxime chloride also slows the process of “aging” of phosphorylated cholinesterase to a nonreactivatable form, and detoxifies certain organophosphates by direct chemical reaction. The drug has its most critical effect in relieving paralysis of the muscles of respiration. Because pralidoxime chloride is less effective in relieving depression of the respiratory center, atropine is always required concomitantly to block the effect of accumulated acetylcholine at this site. Pralidoxime chloride relieves muscarinic signs and symptoms, salivation, bronchospasm, etc., but this action is relatively unimportant since atropine is adequate for this purpose.
PROTOPAM Chloride has been studied in animals as an antidote against numerous organophosphate pesticides, chemicals, and drugs (see Animal Pharmacology and Toxicology). Regardless of whether or not animal studies suggest that the organophosphate poison to which a particular patient has been exposed is amenable to treatment with pralidoxime chloride, the use of pralidoxime chloride should, nevertheless, be considered in any life-threatening situation resulting from poisoning by these compounds, since the limited and arbitrary conditions of pharmacologic screening do not always accurately reflect the usefulness of pralidoxime chloride in the clinical situation.
-
CLINICAL STUDIES
There are no adequate and well controlled clinical studies that establish the effectiveness of pralidoxime chloride as a treatment for poisoning with organophosphates having anticholinesterase activity. However, its use has been considered to be successful against poisoning with numerous pesticides, chemicals, and drugs.
Pharmacokinetics
Animal studies suggest that the minimum therapeutic concentration of pralidoxime in plasma is 4 µg/mL; this level is reached in about 16 minutes after a single injection of 600 mg pralidoxime chloride. In one study of healthy adult volunteers and patients self-poisoned with organophosphate compounds, a single intramuscular injection of 1000 mg of pralidoxime chloride resulted in mean peak plasma levels of 7.5 ± 1.7 µg/mL and 9.9 ± 2.4 µg/mL, respectively. Time to reach the mean peak plasma levels in both groups was similar, 34 minutes in healthy adults and 33 minutes in poisoned patients. Mean half-life was about 3 hours in both groups.
Some evidence suggests that a loading dose followed by continuous intravenous infusion of pralidoxime chloride may maintain therapeutic levels longer than short intermittent infusion therapy. In a cross-over study of seven healthy adults (18 – 50 years) a short intravenous infusion dose of 16 mg/kg over 30 minutes was compared to an intravenous loading dose of 4 mg/kg over 15 minutes, followed by 3.2 mg/kg/hr for 3.75 hours (for a total dose of 16 mg/kg). Results showed that the mean time over which plasma levels were maintained above 4 µg/mL was prolonged in the volunteers who received a loading dose followed by continuous infusion as compared to those who received short infusion therapy (257.5 ± 50.5 min vs. 118.0 ± 52.1 min). Use of continuous intravenous infusion in adult patients with organophosphate poisoning has been described in several case reports, with and without loading doses. Infusion rates ranged from 400 – 600 mg/hr. In one case the blood levels were 11.6 – 13.7 µg /mL when given 400 mg/hr over 5 days (measured at 5, 10 and 18 hours). In another case following an initial loading dose of 1000 mg, blood levels were 11.79 µg/mL when given 500 mg/hr and 17.26 µg/mL when given 600 mg/hr. In the latter case the pralidoxime elimination half-life was 4 hours. In two other cases blood levels were not measured.
Pralidoxime chloride is distributed throughout the extracellular water; its apparent volume of distribution at steady state has been reported to range from 0.60 to 2.7 L/kg. Pralidoxime chloride is not bound to plasma protein.
Pralidoxime chloride is relatively short acting and repeated doses may be needed, unless continuous intravenous infusion is selected. Simulations suggest that after a dose of 1000 mg given intravenously, concentrations fall below 4 µg/mL in about 1.5 hours. The short duration of action of pralidoxime chloride and the necessity for repeated doses should be considered especially where there is any evidence of continuing absorption of the poison. The apparent half-life of pralidoxime chloride is 74 to 77 minutes. The drug is rapidly excreted in the urine by renal tubular secretion, partly unchanged, and partly as a metabolite produced by the liver. After intramuscular administration of 1000 mg of pralidoxime chloride, the renal clearance has been reported to be 7.2 ± 2.9 mL/min/kg in healthy volunteers and 3.6 ± 1.5 mL/min/kg in organophosphate-poisoned patients.
In one study of 11 organophosphate-poisoned pediatric patients (age, 0.8 to 18 years), an intravenous loading dose of 15-50 mg/kg (mean 29 mg/kg) of pralidoxime chloride followed by a continuous infusion of 10-16 mg/kg/hr (mean 14 mg/kg/hr) over 12 to 43 hours (mean 27 ± 8 hours) resulted in an average steady state plasma concentration of 22.2 mg/L (6.9 to 47.4 mg/L) and an average body clearance of 0.88 L/kg/hr (0.28 to 2.20 L/kg/hr). After the continuous infusion was discontinued, determinations of the apparent volume of distribution and half-life ranged from 1.7 to 13.8 L/kg and from 2.4 to 5.3 hours, respectively.
-
INDICATIONS AND USAGE
PROTOPAM Chloride is indicated as an antidote:
- 1. In the treatment of poisoning due to those pesticides and chemicals (e.g., nerve agents) of the organophosphate class which have anticholinesterase activity and
- 2. In the control of overdosage by anticholinesterase drugs used in the treatment of myasthenia gravis.
The principal indications for the use of PROTOPAM Chloride are muscle weakness and respiratory depression. In severe poisoning, respiratory depression may be due to muscle weakness.
-
CONTRAINDICATIONS
There are no known absolute contraindications for the use of PROTOPAM Chloride (see PRECAUTIONS, Drug Interactions and DOSAGE AND ADMINISTRATION). Relative contraindications include known hypersensitivity to the drug and other situations in which the risk of its use clearly outweighs possible benefit.
-
WARNINGS
PROTOPAM Chloride is not effective in the treatment of poisoning due to phosphorus, inorganic phosphates, or organophosphates not having anticholinesterase activity.
PROTOPAM Chloride is not indicated as an antidote for intoxication by pesticides of the carbamate class since it may increase the toxicity of carbaryl.
-
PRECAUTIONS
General
PROTOPAM Chloride has been well tolerated in most cases, but it must be remembered that the desperate condition of the organophosphate-poisoned patient will generally mask such minor signs and symptoms as have been noted in normal subjects.
Intravenous administration of PROTOPAM Chloride should be carried out slowly and, preferably, by continuous or intermittent infusion, since temporary worsening of cholinergic manifestations (i.e. tachycardia, cardiac arrest, laryngospasm, and muscle rigidity or paralysis) may occur if PROTOPAM Chloride is infused too rapidly. The intermittent infusion rate should not exceed 200 mg/minute. If intravenous administration is not feasible, intramuscular or subcutaneous injection should be used (see DOSAGE AND ADMINISTRATION).
PROTOPAM Chloride should be used with great caution in treating organophosphate overdosage in cases of myasthenia gravis since it may precipitate a myasthenic crisis.
Because pralidoxime is excreted in the urine, a decrease in renal function will result in increased blood levels of the drug. Thus, the dosage of PROTOPAM Chloride should be reduced in the presence of renal insufficiency.
Laboratory Tests
Treatment of organophosphate poisoning should be instituted without waiting for the results of laboratory tests. Red blood cell, plasma cholinesterase, and urinary paranitrophenol measurements (in the case of parathion exposure) may be helpful in confirming the diagnosis and following the course of the illness, although such tests may be normal in the face of clinically significant organophosphate poisoning. A reduction in red blood cell cholinesterase concentration to below 50% of normal has been seen only with organophosphate ester poisoning.
Drug Interactions
When atropine and pralidoxime chloride are used together, the signs of atropinization (flushing, mydriasis, tachycardia, dryness of the mouth and nose) may occur earlier than might be expected when atropine is used alone. This is especially true if the total dose of atropine has been large and the administration of pralidoxime chloride has been delayed.
The following precautions should be kept in mind in the treatment of anticholinesterase poisoning, although they do not bear directly on the use of pralidoxime chloride: since barbiturates are potentiated by the anticholinesterases, they should be used cautiously in the treatment of convulsions; morphine, theophylline, aminophylline, reserpine, and phenothiazine-type tranquilizers should be avoided in patients with organophosphate poisoning. Prolonged paralysis has been reported in patients when succinylcholine is given with drugs having anticholinesterase activity; therefore, it should be used with caution.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Because pralidoxime chloride is indicated for short-term emergency use only, no investigations of its potential for carcinogenesis, mutagenesis, or impairment of fertility have been conducted by the manufacturer, or reported in the literature.
Pregnancy
Teratogenic Effects:
Animal reproduction studies have not been conducted with pralidoxime chloride. It is also not known whether pralidoxime chloride can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Pralidoxime chloride should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when pralidoxime chloride is administered to a nursing woman.
Pediatric Use
There are no adequate and well-controlled clinical trials that establish the effectiveness of pralidoxime chloride in pediatric patients. Efficacy has been extrapolated from the adult population and is supported by nonclinical studies, pharmacokinetic studies in adults and experience in the pediatric population (see DOSAGE AND ADMINISTRATION). As in adults, laryngospasm, cardiac arrest, tachycardia, and muscle rigidity or paralysis have been reported following rapid intravenous injection. Muscle fasciculations, apnea, and convulsions have also been reported.
Geriatric Use
Clinical studies of PROTOPAM Chloride did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
ADVERSE REACTIONS
Forty to 60 minutes after intramuscular injection, mild to moderate pain may be experienced at the site of injection.
Pralidoxime chloride may cause blurred vision, diplopia and impaired accommodation, dizziness, headache, drowsiness, nausea, tachycardia, increased systolic and diastolic blood pressure, hyperventilation, and muscular weakness when given parenterally to normal volunteers who have not been exposed to anticholinesterase poisons. In patients, it is very difficult to differentiate the toxic effects produced by atropine or the organophosphate compounds from those of the drug.
Elevations in SGOT and/or SGPT enzyme levels were observed in 1 of 6 normal volunteers given 1200 mg of pralidoxime chloride intramuscularly, and in 4 of 6 volunteers given 1800 mg intramuscularly. Levels returned to normal in about 2 weeks. Transient elevations in creatine phosphokinase were observed in all normal volunteers given the drug.
When atropine and pralidoxime chloride are used together, the signs of atropinization may occur earlier than might be expected when atropine is used alone. This is especially true if the total dose of atropine has been large and the administration of pralidoxime chloride has been delayed. Excitement and manic behavior immediately following recovery of consciousness have been reported in several cases. However, similar behavior has occurred in cases of organophosphate poisoning that were not treated with pralidoxime chloride.
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
Organophosphate Poisoning
Treatment should include general supportive care, atropinization, and decontamination, in addition to the use of PROTOPAM Chloride. Treatment is most effective if initiated immediately after poisoning. Administration of PROTOPAM Chloride should be carried out slowly and, preferably, by infusion. If intravenous administration is not feasible, intramuscular or subcutaneous injection should be used. Generally, little is accomplished if PROTOPAM Chloride is given more than 36 hours after termination of exposure to the poison. When the poison has been ingested, it is particularly important to take into account the likelihood of continuing absorption from the lower bowel since this constitutes new exposure and fatal relapses have been reported after initial improvement. In such cases, additional doses of PROTOPAM Chloride may be needed every three to eight hours. In effect, the patient should be “titrated” with PROTOPAM Chloride as long as signs of poisoning recur. As in all cases of organophosphate poisoning, care should be taken to keep the patient under observation for at least 48 to 72 hours.
If dermal exposure has occurred, clothing should be removed and the hair and skin washed thoroughly with sodium bicarbonate or alcohol as soon as possible.
Supportive care, including airway management, respiratory and cardiovascular support, correction of metabolic abnormalities, and seizure control, may be necessary in cases of severe organophosphate poisoning.
Atropine should be given as soon as possible after hypoxemia is improved. Atropine should not be given in the presence of significant hypoxia due to the risk of atropine-induced ventricular fibrillation. In adults, atropine may be given intravenously in doses of 2 to 4 mg. This should be repeated at 5- to 10-minute intervals until full atropinization (secretions are inhibited) or signs of atropine toxicity appear (delirium, hyperthermia, muscle twitching).
Some degree of atropinization should be maintained for at least 48 hours, and until any depressed blood cholinesterase activity is reversed.
Use of morphine, theophylline, aminophylline, reserpine, and phenothiazine-type tranquilizers should be avoided in patients with organophosphate poisoning (see PRECAUTIONS, Drug Interactions). Prolonged paralysis has been reported in patients when succinylcholine is given with drugs having anticholinesterase activity; therefore, it should be used with caution.
After the effects of atropine become apparent, PROTOPAM Chloride may be administered.
Symptoms Of Nerve Agent And Insecticide Poisoning
PROTOPAM Chloride dosing is based, in part, on the severity of symptoms of nerve agent intoxication. These symptoms include the following:
MILD symptoms:
- Blurred vision and sore eyes
- Teary eyes*
- Runny nose*
- Increased salivation such as sudden drooling*
- Chest tightness or difficulty breathing
- Tremors throughout the body or muscular twitching
- Nausea and vomiting
- Involuntary respiratory secretions
SEVERE symptoms:
- Strange or confused behavior
- Severe difficulty breathing or respiratory secretions
- Severe muscular twitching and general weakness**
- Involuntary urination and defecation*
- Convulsions
- Unconsciousness
Symptoms in INFANTS AND YOUNG CHILDREN:
* These symptoms are sometimes observed in healthy infants and young children. In this age group, these symptoms are less reliable than other symptoms listed. Symptoms must be considered collectively when nerve agent or pesticide exposure is known or suspected.
** Infants may become drowsy or unconscious, with muscle floppiness rather than muscle twitching, soon after exposure to nerve agents or pesticides.
ADULT DOSING
ADULT INTRAVENOUS DOSING:
Refer to the Preparation for Administration section for instructions on reconstitution and dilution of PROTOPAM Chloride that result in a 10-20 mg/mL solution for intravenous infusion.
Inject an initial dose of 1000 to 2000 mg of PROTOPAM Chloride, preferably as an infusion in 100 mL of normal saline, over a 15- to 30-minute period. If this is not practical or if pulmonary edema is present, the dose should be given slowly (over not less than five minutes) by intravenous injection, as a 50 mg/mL solution in water (e.g., 1000 mg in 20 mL). A second dose of 1000 to 2000 mg may be indicated after about one hour if muscle weakness has not been relieved. Additional doses may be given every 10-12 hours if muscle weakness persists.
Intravenous administration of PROTOPAM Chloride should be carried out slowly and, preferably, by continuous or intermittent infusion, since temporary worsening of cholinergic manifestations (i.e. tachycardia, cardiac arrest, laryngospasm, and muscle rigidity or paralysis) may occur if PROTOPAM Chloride is infused too rapidly. The intermittent infusion rate should not exceed 200 mg/minute. If intravenous administration is not feasible, intramuscular or subcutaneous injection should be used.
Evidence suggests that a loading dose followed by continuous intravenous infusion of PROTOPAM Chloride may maintain therapeutic levels longer than traditional short intermittent infusions therapy (see Pharmacokinetics).
ADULT INTRAMUSCULAR DOSING:
Refer to the Preparation for Administration section for instructions on reconstitution of PROTOPAM Chloride that result in an approximate 300 mg/mL solution for intramuscular administration.
Intramuscular dosing in adults should be based on the severity of clinical symptoms.
MILD SYMPTOMS
- For treatment of mild symptoms, administer a 600 mg (2 mL) intramuscular dose of PROTOPAM Chloride. Wait 15 minutes for PROTOPAM Chloride to take effect.
- If, after 15 minutes, mild symptoms persist, then administer a second 600 mg (2 mL) intramuscular dose of PROTOPAM Chloride.
- If, after an additional 15 minutes, mild symptoms continue to persist, a third 600 mg (2 mL) dose of PROTOPAM Chloride may be administered for a total cumulative dose of 1800 mg.
- If at any time after the first dose, the patient develops severe symptoms, administer two additional 600 mg intramuscular doses in rapid succession for a total cumulative dose of 1800 mg of PROTOPAM Chloride.
SEVERE SYMPTOMS
- For treatment of severe symptoms, administer three 600 mg intramuscular doses (3 doses of 2 mL each) in rapid succession for a total dose of 1800 mg of PROTOPAM Chloride.
PERSISTENT SYMPTOMS
- If symptoms persist after administering the complete 1800 mg regimen (3 injections of 600 mg each), the series may be repeated beginning approximately 1 hour after administration of the last injection.
PEDIATRIC DOSING (FOR PATIENTS 16 YEARS AND UNDER)
PEDIATRIC INTRAVENOUS DOSING:
Refer to the Preparation for Administration section for instructions on reconstitution and dilution of PROTOPAM Chloride that result in 10-20 mg/mL solution for intravenous infusion.
PROTOPAM Chloride can be given as intermittent intravenous infusions or as a loading dose followed by continuous intravenous infusion, depending upon the patient’s clinical condition. The specific dose given should depend upon the severity of the symptoms.
Loading Dose Following By Continuous Infusion:
Administer a loading dose of 20-50 mg/kg (not to exceed 2000 mg/dose) over 15-30 minutes followed by a continuous infusion of 10-20 mg/kg/hour.
Intermittent Infusion Dosing:
Administer an initial intermittent infusion of 20-50 mg/kg (not to exceed 2000 mg/dose) over 15-30 minutes. A second dose of 20-50 mg/kg may be indicated after about one hour if muscle weakness has not been relieved. Repeat dosing is permissible every 10-12 hours as needed.
If it is not practical to administer intermittent or continuous intravenous infusions, or if pulmonary edema is present, the 20-50 mg/kg dose should be given slowly (over not less than five minutes) by intravenous injection as a 50 mg/mL solution in water (see Preparation for Administration section). Additional doses may be given every 10-12 hours if muscle weakness persists.
PEDIATRIC INTRAMUSCULAR DOSING:
Refer to the Preparation for Administration section for instructions on reconstitution of PROTOPAM Chloride that result in an approximate 300 mg/mL solution for intramuscular administration.
Intramuscular injections in children should be administered in the anterolateral aspect of the thigh to avoid the nerve, artery and vein, as well as the femur.
Pharmacokinetic modeling using published data from the scientific literature was conducted to derive intramuscular dosing recommendations in the pediatric population. The specific intramuscular dose of PROTOPAM Chloride should depend upon the severity of the symptoms.
MILD SYMPTOMS
- For the treatment of mild symptoms, administer a weight-appropriate intramuscular dose (see Table 1 below) of PROTOPAM Chloride. Wait 15 minutes for PROTOPAM Chloride to take effect.
- If, after 15 minutes, mild symptoms persist, then administer a second weight-appropriate intramuscular dose of PROTOPAM Chloride.
- If after an additional 15 minutes, mild symptoms continue to persist, a third weight-appropriate intramuscular dose of PROTOPAM Chloride may be administered.
- The three PROTOPAM Chloride injections together are considered a single course of treatment, and the total amount of PROTOPAM Chloride administered per course of treatment (i.e., 3 weight-appropriate injections) should not exceed the total amounts listed in Table 1 below.
- If at any time after the first dose, the patient develops severe symptoms, administer two additional weight-appropriate intramuscular doses of PROTOPAM Chloride in rapid succession.
SEVERE SYMPTOMS
- For treatment of severe symptoms, administer the weight-appropriate intramuscular dose (see Table 1 below) of PROTOPAM Chloride Injection as three injections, in rapid succession, into the patient’s anterolateral thigh (see Table 1 below).
PERSISTENT SYMPTOMS
- If symptoms persist after administering a complete course (3 injections of the weight-appropriate dose each), the series may be repeated beginning approximately 1 hour after administration of the last injection.
Table 1: Pediatric Intramuscular Dosing Recommendations* - * Dosing is based on an approximate 300 mg/mL solution.
- † During the treatment of mild symptoms, if at any time after the first dose, the patient develops severe symptoms, administer two additional weight-appropriate intramuscular doses of PROTOPAM Chloride in rapid succession.
- ‡ Additional courses of PROTOPAM Chloride may be administered beginning one hour after the last injection. A single course consists of three separate, weight-appropriate injections, administered either with 15 minute inter-injection observation periods for patients with mild symptoms, or all in rapid succession for patients with severe symptoms.
- § Weight of 40 kg corresponds to approximately the 50th percentile for a 12 year old child per the weight-for-age percentile growth charts published by the Centers for Disease Control and Prevention in 2000.
- ¶ Adult Dose Per Injection is 600 mg; Total Adult Dose per Three-Injection Course is 1800 mg.
Weight in kg
Dose Per Injection†
Total Dose per Three-Injection Course‡
< 40kg
15 mg/kg
45 mg/kg
≥ 40 kg§
Use Adult Dosing Recommendations¶
Use Adult Dosing Recommendations
Anticholinesterase Overdosage
As an antagonist to such anticholinesterases as neostigmine, pyridostigmine, and ambenonium, which are used in the treatment of myasthenia gravis, PROTOPAM Chloride may be given in a dosage of 1000 to 2000 mg intravenously followed by increments of 250 mg every five minutes.
Preparation for Administration
PROTOPAM Chloride is supplied as 1000 mg single-dose vials for injection.
For INTRAVENOUS infusion: Reconstitute a single PROTOPAM Chloride 1000 mg vial by adding 20 mL of Sterile Water for Injection, USP, which results in a 50 mg/mL concentration.
The solution should further be diluted with Normal Saline for Injection, USP to achieve a concentration of 10 to 20 mg/mL (e.g. 1000 mg in 100 mL or 2000 mg in 100 mL).
For fluid restricted patients or for rapid administration (over at least 5 min), a maximum concentration of 50 mg/mL may be used.
For INTRAMUSCULAR injection: Reconstitute a single PROTOPAM Chloride 1000 mg vial by adding 3.3 mL of Sterile Water for Injection, USP for an approximate concentration of 300 mg/mL.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Discard unused solution after a dose has been withdrawn.
-
HOW SUPPLIED
NDC: 60977-141-01—Hospital Package: This contains six 20 mL vials of 1 g each of sterile PROTOPAM Chloride (pralidoxime chloride) for Injection white to off-white porous cake*, without diluent or syringe.
*When necessary, sodium hydroxide is added during processing to adjust the pH.
-
ANIMAL PHARMACOLOGY AND TOXICOLOGY
The following table lists chemical and trade or generic names of pesticides, chemicals, and drugs against which PROTOPAM Chloride (usually administered in conjunction with atropine) has been found to have antidotal activity on the basis of animal experiments. All compounds listed are organophosphates having anticholinesterase activity. A great many additional substances are in industrial use but have been omitted because of lack of specific information.
AAT—see PARATHION
AFLIX—see FORMOTHION
ALKRON—see PARATHION
AMERICAN CYANAMID 3422—see PARATHION
AMITON—diethyl-S-(2-diethylaminoethyl)phosphorothiolate
ANTHIO—see FORMOTHION
APHAMITE—see PARATHION
ARMIN—ethyl-4-nitrophenylethylphosphonate
AZINPHOS-METHYL—dimethyl-S-[(4-oxo-1,2,3,-benzotriazin-3(4 H)-yl)methyl] phosphorodithioate
MORPHOTHION—dimethyl-S-2-keto-2-(N-morpholyl)ethylphosphorodithioate
NEGUVON—see TRICHLOROFON
NIRAN—see PARATHION
NITROSTIGMINE—see PARATHION
O,O-DIETHYL-O-p-NITROPHENYL PHOSPHOROTHIOATE—see PARATHION
O,O-DIETHYL-O-p-NITROPHENYLTHIO PHOSPHATE—see PARATHION
OR 1191—see PHOSPHAMIDON
OS 1836—see VINYLPHOS
OXYDEMETONMETHYL—dimethyl-S-2-(ethylsulfinyl) ethyl phosphorothiolate
PARAOXON—diethyl (4-nitrophenyl) phosphate
PARATHION—diethyl (4-nitrophenyl) phosphorothionate
PENPHOS—see PARATHION
PHENCAPTON—diethyl-S-(2,5-dichlorophenylmercaptomethyl) phosphorodithioate
PHOSDRIN—see MEVINPHOS
PHOS-KIL—see PARATHION
PHOSPHAMIDON—1-chloro-1-diethylcarbamoyl-1-propen-2-yl-dimethylphosphate
PHOSPHOLINE IODIDE—see echothiophate iodide
PHOSPHOROTHIOIC ACID, O,O-DIETHYL-O-p-NITROPHENYL ESTER—see PARATHION
PLANTHION—see PARATHION
QUELETOX—see FENTHION
RH—see PARATHION
RU—4-tert-butyl-2-chlorophenylmethyl-N-methylphosphoroamidate
SARIN—isopropyl-methylphosphonofluoridate
SHELL OS 1836—see VINYLPHOS
SHELL 2046—see MEVINPHOS
SNP—see PARATHION
SOMAN - pinacolyl-methylphosphonofluoridate
SYSTOX - diethyl-(2-ethylmercaptoethyl) phosphorothionate
TEP - see TEPP
TEPP - tetraethylpyro phosphate
THIOPHOS- see PARATHION
TIGUVON - see FENTHION
TRICHLOROFON - dimethyl-1-hydroxy-2,2,2-trichloroethylphosphonate
VAPONA - see DICHLORVOS
VAPOPHOS - see PARATHION
VINYLPHOS - diethyl-2-chloro-vinylphosphatePROTOPAM Chloride (pralidoxime chloride) appears to be ineffective, or marginally
effective, against poisoning by:CIODRIN (alpha-methylbenzyl-3-[dimethoxyphosphinyloxy]-ciscrotonate)
DIMEFOX (tetramethylphosphorodiamidic fluoride)
DIMETHOATE (dimethyl-S-[N-methylcarbamoylmethyl]phosphorodithioate)
METHYL DIAZINON (dimethyl-[2-isopropyl-4-methylpyrimidyl]-phosphorothionate)
METHYL PHENCAPTON (dimethyl-S-[2,5-dichlorophenylmercaptomethyl]phosphorodithioate)
PHORATE (diethyl-S-ethylmercaptomethylphosphorodithioate)
SCHRADAN (octamethylpyrophosphoramide)
WEPSYN (5-amino-1-[bis-(dimethylamino) phosphinyl]-3-phenyl-1,2,4-triazole). -
SPL UNCLASSIFIED SECTION
Baxter and Protopam are trademarks of Baxter International Inc.
All other trademarks or brand names appearing herein are the property of their respective owners.Manufactured for
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
By: Baxter Pharmaceutical Solutions LLC Bloomington, IN 47403For Product Inquiry 1 800 ANA DRUG (1-800-262-3784)
07-19-00-0141
Rev: January 2018
-
PACKAGE LABEL - Principal Display Panel
Container Label
NDC: 60977-141-27
Protopam Chloride
(pralidoxime chloride) for injection
1 g Single Dose Vial
Rx only
FOR INTRAVENOUS INJECTION;
may be given subcutaneously or
intramuscularly if this route is not feasible.
Prepare injection by adding
20 mL of Sterile Water for
Injection, USP.
Usual Dosage: 1 g
See accompanying descriptive
literature. Discard unused solution
after a dose have been withdrawn.
Prepared by cryodesiccation.
esi
Mfd. for Baxter Healthcare Corp.
Deerfield, IL 60015 USA
by: Baxter Pharmaceutical Solutions, LLC
Bloomington, IN 47403
460-496-01
(01)00360977141275
3-942-233
Lot:
Exp:
Carton Label
NDC: 60977-141-01
Protopam Chloride
(pralidoxime chloride) for injection
1 g
Rx only
6 x 1 g Single Dose Vials
FOR INTRAVENOUS INJECION;
may also be given by intramuscular or subcutaneous
injection if the intravenous route is not feasible.
esi
Manufactured for
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
by: Baxter Pharmaceutical Solutions, LLC
Bloomington, IN 47403
Lot:
Exp:
NDC: 60977-141-01
Protopam Chloride
(pralidoxime chloride) for injection
1 g
6 x 1 g Single Dose Vials
N3 60977 14101 5
Enclosed in this package are six vials, each containing 1 g of sterile
Protopam Chloride (pralidoxime chloride). When necessary, either 1N
hydrochloric acid or 1N sodium hydroxide may be added during
processing to adjust the pH. Prepare injection by adding 20 mL of
Sterile Water for Injection, USP (not supplied in this package).
Usual Dosage: 1 g
See accompanying descriptive literature.
Discard unused solution after a dose has been withdrawn.
Prepared by cryodesiccation.
Enclosed product circular for human use.
Store at 20°-25°C (68°-77°F) [see USP Controlled Room Temperature].
Baxter and Protopam are trademarks of Baxter International Inc. or its subsidiaries.
460-497-00
3-809-1808
NDC: 60977-141-01
Protopam Chloride
(pralidoxime chloride) for injection
1 g
6 x 1 g Single Dose Vials
FOR INTRAVENOUS INJECTION;
may also be given by intramuscular
or subcutaneous injection
if the intravenous route is not feasible.
esi
Manufactured for
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
by: Baxter Pharmaceutical Solutions LLC
Bloomington, IN 47403
PROTOPAM Chloride
(pralidoxime chloride) for injection1 gram
Store at 20° - 25°C (68° - 77°F)
EXP 06 2023 LOT 933462
Bar code
(17) 230600 (10) 933462Bar code
(01) 5 0360977 14101 0 (30) 0276Baxter Healthcare Corporation
Baxter Pharmaceutical Solutions LLC
Bloomington, IN 47403NDC: 60977-141-01
46 Packs, each containing
6 X 20 mL VialsBar code
(01) 5 0360977 14101 0
(21) 000000000493
(17) 230616
(10) 93346207-06-77-165
Bar code
20-4682-233 -
INGREDIENTS AND APPEARANCE
PROTOPAM CHLORIDE
pralidoxime chloride injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 60977-141 Route of Administration INTRAVENOUS, INTRAMUSCULAR, SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PRALIDOXIME CHLORIDE (UNII: 38X7XS076H) (PRALIDOXIME - UNII:P7MU9UTP52) PRALIDOXIME CHLORIDE 1 g in 20 mL Inactive Ingredients Ingredient Name Strength SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 60977-141-01 6 in 1 PACKAGE 03/10/1965 1 NDC: 60977-141-27 20 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA014134 03/10/1965 Labeler - Baxter Healthcare Corporation (005083209) Establishment Name Address ID/FEI Business Operations BAXTER PHARMACEUTICAL SOLUTIONS, LLC 604719430 ANALYSIS(60977-141) , MANUFACTURE(60977-141) , PACK(60977-141) , LABEL(60977-141) , STERILIZE(60977-141)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.