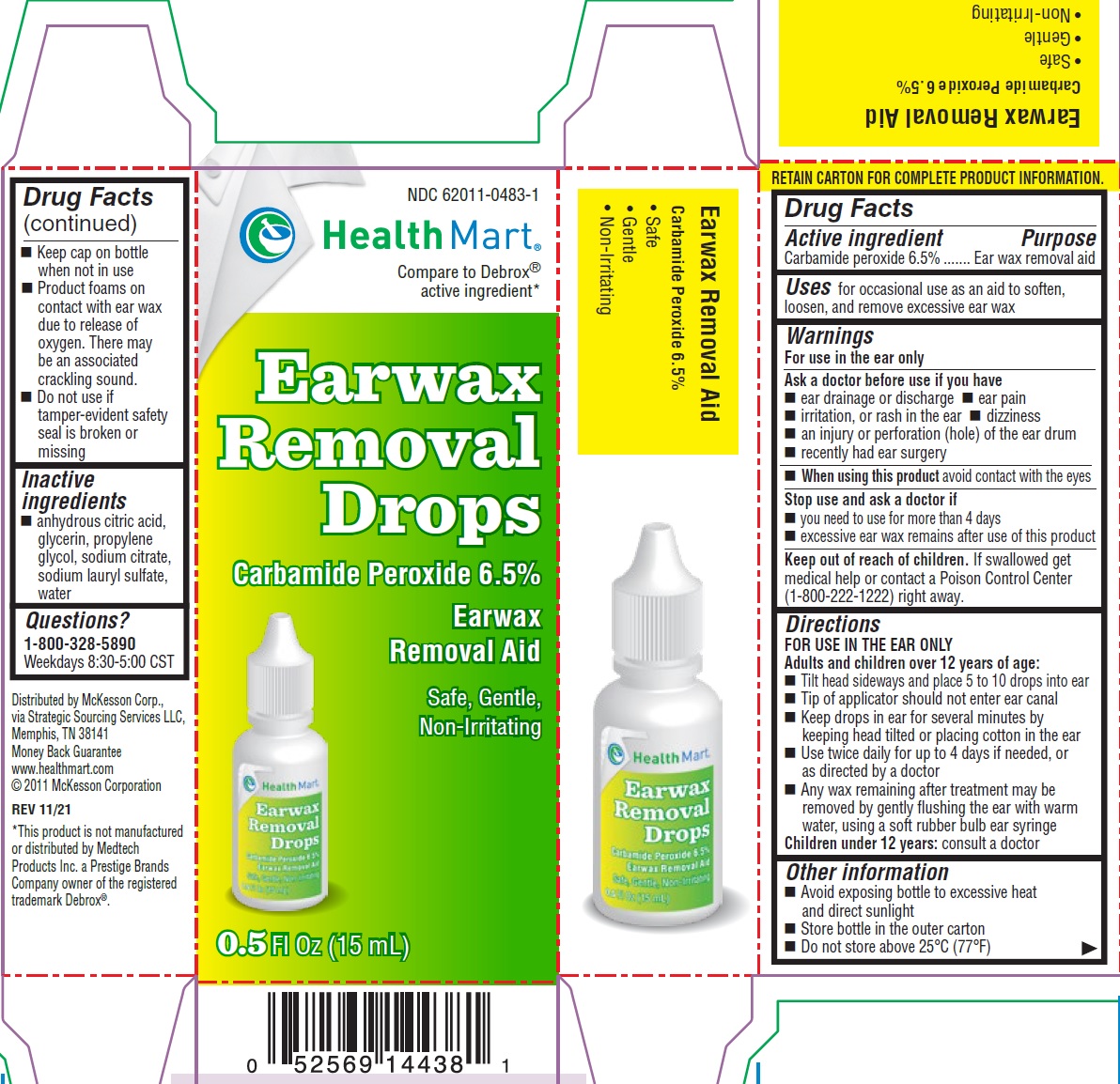
HealthMart Ear Wax Removal Drops
HealthMart Ear Wax Removal Drops by
Drug Labeling and Warnings
HealthMart Ear Wax Removal Drops by is a Otc medication manufactured, distributed, or labeled by McKesson Corp via Strategic Sourcing Services LLC, Bell Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
HEALTHMART EAR WAX REMOVAL DROPS- carbamide peroxide solution/ drops
McKesson Corp via Strategic Sourcing Services LLC
----------
HealthMart Ear Wax Removal Drops
Warnings
For use in the ear only
Ask a doctor before use if you have
ear drainage or discharge ear pain irritation, or rash in the ear dizziness an injury or perforation (hole) of the ear drum recently had ear surgery
Directions
FOR USE IN THE EAR ONLY
Adults and children over 12 years of age:
consult a doctor Children under 12 years:
- Tilt head sideways and place 5 to 10 drops into ear
- Tip of applicator should not enter ear canal
- Keep drops in ear for several minutes bykeeping head tilted or placing cotton in the ear
- Use twice daily for up to 4 days if needed, or as directed by a doctor
- Any wax remaining after treatment may be removed by gently flushing the ear with warm water, using a soft rubber bulb ear syringe
Other information
- Avoid exposing bottle to excessive heat and direct sunlight
- Store bottle in the outer carton
- Do not store above 25°C (77°F)
- Keep cap on bottle when not in use
- Product foams on contact with ear wax due to release of oxygen. There may be an associated crackling sound.
- Do not use if tamper-evident safety seal is broken or missing
| HEALTHMART EAR WAX REMOVAL DROPS
carbamide peroxide solution/ drops |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - McKesson Corp via Strategic Sourcing Services LLC (116956644) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bell Pharmaceuticals, Inc. | 140653770 | manufacture(62011-0483) | |
Revised: 1/2025
Document Id: 2cdf4b71-590f-d318-e063-6294a90aab09
Set id: 27b9b9d4-d8c3-43d8-915b-ebcd0f0c0da0
Version: 3
Effective Time: 20250129
McKesson
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.