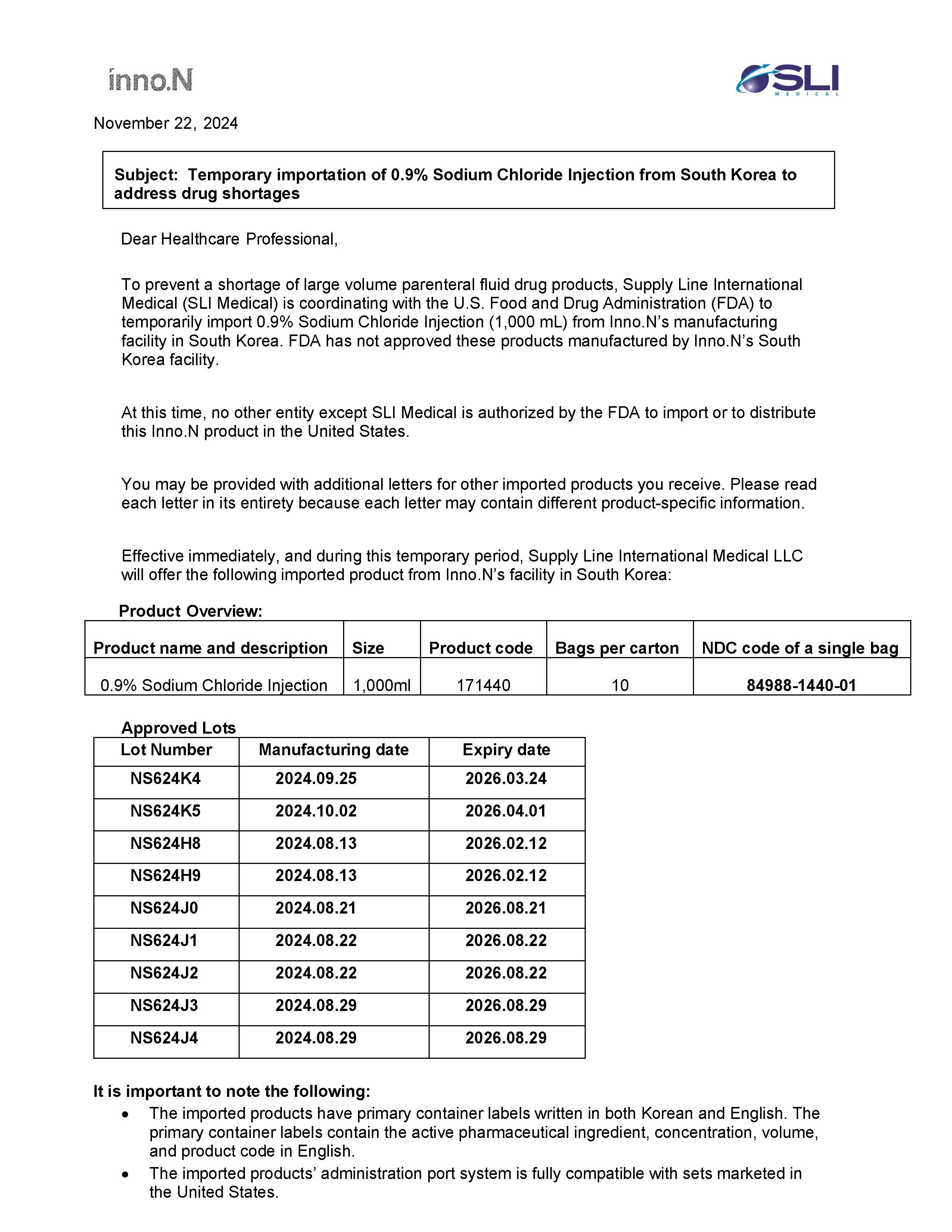
Sodium Chloride Injection, solution by HK INNO.N CORPORATION
Sodium Chloride Injection, solution by
Drug Labeling and Warnings
Sodium Chloride Injection, solution by is a Prescription medication manufactured, distributed, or labeled by HK INNO.N CORPORATION. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SODIUM CHLORIDE INJECTION, SOLUTION- sodium chloride injection injection
HK INNO.N CORPORATION
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
INDICATIONS & USAGE
Sodium Chloride Injection, USP is indicated as a source of water and electrolytes. 0.9% Sodium Chloride Injection, USP is also indicated for use as a priming solution in hemodialysis procedures.
Storage: Store at room temperature 20°C/68°F. to 25°C/77°F.
Medication Administration: Contains medication port and administration port; Twist off shield, left side
These highlights do not include all the information needed to use Sodium Chloride 0.9% Injection. See full prescribing information for Chloride 0.9% Injection. This product is an unapproved product for use in urgent drug shortage” (code C101533).
| SODIUM CHLORIDE INJECTION, SOLUTION
sodium chloride injection injection |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - HK INNO.N CORPORATION (689049556) |
| Registrant - HK INNO.N CORPORATION (689049556) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| HK INNO.N CORPORATION | 689049556 | manufacture(84988-1440) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.