RAPIBLYK- landiolol injection, powder, lyophilized, for solution
RAPIBLYK by
Drug Labeling and Warnings
RAPIBLYK by is a Prescription medication manufactured, distributed, or labeled by AOP Orphan Pharmaceuticals GmbH. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use RAPIBLYK safely and effectively. See full prescribing information for RAPIBLYK.
RAPIBLYK (landiolol) for injection, for intravenous use
Initial U.S. Approval: 2024INDICATIONS AND USAGE
RAPIBLYK is a beta adrenergic blocker indicated for the short-term reduction of ventricular rate in adults with supraventricular tachycardia including atrial fibrillation and atrial flutter. ( 1)
DOSAGE AND ADMINISTRATION
- Administer as an intravenous infusion in a monitored setting. ( 2.1)
- Titrate according to ventricular rate. ( 2.1)
- If normal cardiac function, start at 9 mcg/kg/min; adjust dose in 10-minute intervals as needed in increments of 9 mcg/kg/min to a maximum of 36 mcg/kg/min. ( 2.1)
- If impaired cardiac function, start at 1 mcg/kg/min; adjust dose in 15-minute intervals as needed in increments of 1 mcg/kg/min to a maximum of 36 mcg/kg/min. ( 2.1).
DOSAGE FORMS AND STRENGTHS
For injection: 280 mg of landiolol (equivalent to 300 mg of landiolol HCl) as a lyophilized powder in a single-dose vial. ( 3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Risk of hypotension, bradycardia, and cardiac failure: Monitor for signs and symptoms of cardiovascular adverse effects. Reduce or discontinue use ( 5.1, 5.2, 5.3)
- Risk of exacerbating reactive airway disease ( 5.5)
- Diabetes mellitus: May mask symptoms of hypoglycemia and alter glucose levels; monitor ( 5.6)
- Monitor for signs of myocardial ischemia when abruptly discontinuing in patients with coronary artery disease ( 5.10)
ADVERSE REACTIONS
The most common adverse reaction (9.9%) is hypotension ( 6.1).
To report SUSPECTED ADVERSE REACTIONS, contact AOP Orphan Pharmaceuticals at drugsafety.us@aop-health.com or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Revised: 11/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Transitioning from RAPIBLYK Injection Therapy to Alternative Medications
2.3 Instructions for Preparation
2.4 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypotension
5.2 Bradycardia
5.3 Cardiac Failure
5.4 Reactive Airways Disease
5.5 Use in Patients with Diabetes Mellitus and Hypoglycemia
5.6 Infusion Site Reactions
5.7 Use in Patients with Prinzmetal’s Angina
5.8 Use in Patients with Pheochromocytoma
5.9 Use in Patients with Peripheral Circulatory Disorders
5.10 Abrupt Discontinuation of RAPIBLYK Injection
5.11 Hyperkalemia
5.12 Use in Patients with Metabolic Acidosis
5.13 Use in Patients with Hyperthyroidism
5.14 Use in Patients at Risk of Severe Acute Hypersensitivity Reactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Negative Inotropes and Chronotropes
7.2 Sympathomimetics, Positive Inotropes and Vasoconstrictors
7.3 Catecholamine Depleting Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Pediatric Use
8.4 Geriatric Use
8.5 Hepatic Impairment
10 OVERDOSAGE
10.1 Signs and Symptoms of Overdose
10.2 Treatment Recommendations
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
Administer RAPIBLYK as a continuous intravenous infusion, titrating as needed for heart rate control. There are limited data beyond 24 hours of use.
Table 1: Dosing Normal cardiac function Impaired cardiac function
Starting dose
9 mcg/kg/min 1 mcg/kg/min Titration interval 10 min 15 min Titration step 9 mcg/kg/min 1 mcg/kg/min Maximum dose 36 mcg/kg/min 36 mcg/kg/min 9 mcg/kg/min landiolol is equivalent to 9.6 mcg/kg/min landiolol hydrochloride.
2.2 Transitioning from RAPIBLYK Injection Therapy to Alternative Medications
When transitioning to alternative medications consider the pharmacodynamics of the medication to which the patient is being transitioned and monitor clinical response. If switched to an oral beta-blocker, the dosage of RAPIBLYK can be reduced as follows:
- Ten minutes after administration of the oral beta-blocker, reduce the infusion rate of RAPIBLYK by 50%.
- If satisfactory control is maintained for at least one hour, discontinue RAPIBLYK.
2.3 Instructions for Preparation
- Use appropriate aseptic technique for reconstitution.
- Reconstitute each 280 mg vial of RAPIBLYK with 50 mL of 0.9% Sodium Chloride Injection, USP or 5% Dextrose Injection, USP. Gently swirl to dissolve contents.
- Inspect parenteral drug products for particulate matter and discoloration prior to administration, whenever solution and container permit. The reconstituted solution should be a clear, colorless solution.
- Use immediately. The reconstituted RAPIBLYK solution storage conditions are described in Table 2.
- Discard unused portion.
Table 2: Reconstituted RAPIBLYK Solution Storage and Use Conditions Diluent used to Prepare Solution
Reconstituted RAPIBLYK Solution Storage and Use Conditions 50 mL of 0.9% Sodium Chloride Injection, USP Use within 4 hours at room temperature (25°C, 77°F) 50 mL of 5% Dextrose Injection, USP Use within 48 hours at room temperature (25°C, 77°F) - 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
RAPIBLYK is contraindicated in patients with:
- Severe sinus bradycardia, sick sinus syndrome, heart block greater than first degree [see Warnings and Precautions (5.2)].
- Decompensated heart failure [see Warnings and Precautions (5.3)].
- Cardiogenic shock: May precipitate further cardiovascular collapse and cause cardiac arrest.
- Pulmonary hypertension: May precipitate cardiorespiratory decompensation.
- Hypersensitivity reactions, including anaphylaxis, to landiolol or any of the inactive ingredients
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypotension
Patients with hemodynamic compromise, hypovolemia, or on interacting medications are at increased risk of hypotension. Monitor blood pressure closely, especially if pretreatment blood pressure is low. Reduce or stop RAPIBLYK injection for hypotension then expect the blood pressure effect to wane within 30 minutes.
5.2 Bradycardia
Patients with first-degree atrioventricular block, sinus node dysfunction, or conduction disorders are at increased risk of bradycardia, including sinus pause, heart block, severe bradycardia, and cardiac arrest. Monitor heart rate and rhythm in patients receiving RAPIBLYK injection. Reduce or stop RAPIBLYK injection for bradyarrhythmia.
5.3 Cardiac Failure
Beta-blockers, like RAPIBLYK, can cause depression of myocardial contractility and may precipitate heart failure and cardiogenic shock. At the first sign or symptom of impending cardiac failure, stop RAPIBLYK injection and start supportive therapy [see Overdosage (10)].
5.4 Reactive Airways Disease
Patients with reactive airways disease should, in general, not receive beta-blockers. Because of its relative beta-1 selectivity and titratability, RAPIBLYK injection may be titrated to the lowest possible effective dose. In the event of bronchospasm, stop the infusion immediately; a beta-2 stimulating agent may be administered with appropriate monitoring of ventricular rates.
5.5 Use in Patients with Diabetes Mellitus and Hypoglycemia
Beta-blockers may prevent early warning signs of hypoglycemia, such as tachycardia, and increase the risk for severe or prolonged hypoglycemia at any time during treatment, especially in patients with diabetes mellitus, patients who are fasting (i.e., surgery, not eating regularly, or are vomiting), or children. Monitor for signs and symptoms of hypoglycemia in patients receiving RAPIBLYK.
5.6 Infusion Site Reactions
Infusion site reactions such as pain, swelling and erythema have occurred with the use of RAPIBLYK injection. Avoid infusions into small veins or through a butterfly catheter. If a local infusion site reaction develops, use an alternative infusion site and avoid extravasation.
5.7 Use in Patients with Prinzmetal’s Angina
Beta-blockers may exacerbate anginal attacks in patients with Prinzmetal’s angina because of unopposed alpha receptor–mediated coronary artery vasoconstriction.
5.8 Use in Patients with Pheochromocytoma
If RAPIBLYK injection is used in the setting of pheochromocytoma, administer RAPIBLYK in combination with an alpha-blocker, and only after the alpha-blocker has been initiated. Administration of beta-blockers without opposing alpha blockade in the setting of pheochromocytoma has been associated with a paradoxical increase in blood pressure from the attenuation of beta receptor-mediated vasodilation in skeletal muscle.
5.9 Use in Patients with Peripheral Circulatory Disorders
RAPIBLYK injection may exacerbate peripheral circulatory disorders, such as Raynaud’s disease or syndrome, and peripheral occlusive vascular disease.
5.10 Abrupt Discontinuation of RAPIBLYK Injection
Severe exacerbations of angina, myocardial infarction, and ventricular arrhythmias have been reported in patients with coronary artery disease upon abrupt discontinuation of beta-blocker therapy. Observe patients for signs of myocardial ischemia when discontinuing RAPIBLYK injection.
5.11 Hyperkalemia
Beta-blockers, including RAPIBLYK injection, can cause increases in serum potassium and hyperkalemia. The risk is increased in patients with risk factors such as renal impairment. Intravenous administration of beta-blockers has been reported to cause potentially life-threatening hyperkalemia in hemodialysis patients. Monitor serum electrolytes during therapy with RAPIBLYK injection.
5.12 Use in Patients with Metabolic Acidosis
Beta-blockers have been reported to cause hyperkalemic renal tubular acidosis. Acidosis in general may be associated with reduced cardiac contractility.
5.13 Use in Patients with Hyperthyroidism
Beta-adrenergic blockade may mask certain clinical signs (e.g., tachycardia) of hyperthyroidism. Abrupt withdrawal of beta blockade might precipitate thyroid storm; therefore, monitor patients for signs of thyrotoxicosis when withdrawing beta blocking therapy.
5.14 Use in Patients at Risk of Severe Acute Hypersensitivity Reactions
When using beta-blockers, patients at risk of anaphylactic reactions may be more reactive to allergen exposure (accidental, diagnostic, or therapeutic).
Patients using beta-blockers may be unresponsive to the usual doses of epinephrine used to treat anaphylactic or anaphylactoid reactions [see Drug Interactions (7)].
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Landiolol injection was studied in 19 placebo-controlled clinical trials involving 1,761 patients (in a variety of clinical in-patient settings) with supraventricular tachycardia or at high risk for supraventricular tachycardia. The most important and common adverse reaction is hypotension, which occurred in 9.9% of patients receiving RAPIBLYK vs. 1% in those receiving placebo [see Warnings and Precautions (5.1)].
-
7 DRUG INTERACTIONS
7.1 Negative Inotropes and Chronotropes
Avoid concomitant use of RAPIBLYK with negative inotropes and medications that slow heart rate or cardiac conduction.
Beta-blockers, like RAPIBLYK, can cause depression of myocardial contractility and increase the risk of bradycardia or heart block. Concomitant use of RAPIBLYK with negative inotropes or chronotropes may augment these effects [see Warnings and Precautions (5.2)(5.3)].
7.2 Sympathomimetics, Positive Inotropes and Vasoconstrictors
Beta adrenergic agonists will antagonize the effects of RAPIBLYK and may attenuate the heart rate lowering effects of RAPIBLYK. Positive inotropes and vasoconstrictors may attenuate the heart rate and blood pressure lowering effects of RAPIBLYK.
7.3 Catecholamine Depleting Drugs
Observe patients treated with RAPIBLYK plus a catecholamine depletor (e.g., reserpine, monoamine oxidase inhibitors) for hypotension or marked bradycardia, which may cause vertigo, syncope, or postural hypotension.
Catecholamine depleting drugs may have an additive effect when given with beta-blockers, which may increase the risk of hypotension or marked bradycardia related vertigo, syncope, or postural hypotension [see Warnings and Precautions (5.1)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The available published data on RAPIBLYK use in pregnant women are insufficient to inform a drug associated risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes. Landiolol exposure was limited to a single injection at the time of Cesarean delivery in a small clinical trial. Neonatal bradycardia, hypoglycemia, and respiratory depression have been observed with use of beta-blockers in pregnancy near the time of delivery (see Clinical Considerations). Administration of landiolol to pregnant rats showed distribution of landiolol to the placenta and the fetus. In animal reproduction studies, no embryo-fetal toxicity was observed in rats or rabbits during the period of organogenesis at landiolol exposure in rats approximately 2.7 times human exposure at the maximum recommended human dose (MRHD) (see Data).
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Landiolol crosses the placenta in rats. Neonates born to mothers who are receiving landiolol during pregnancy, may be at risk for hypotension, hypoglycemia, bradycardia, and respiratory depression. Monitor neonates exposed to landiolol during pregnancy and labor for hypotension, hypoglycemia, bradycardia, and respiratory depression and manage accordingly.
Data
Animal Data
Landiolol HCl was administered intravenously to pregnant rats (25, 50, or 100 mg/kg/day from gestation day 7 to 17) and rabbits (25, 50, or 100 mg/kg/day from gestation day 8 to 18). No adverse embryo-fetal effects were observed in rats at the landiolol HCl dose of 25 mg/kg/day, resulting in systemic landiolol exposure (AUC) of approximately 2.7-times the exposure at the MRHD. In rabbits, no adverse embryo-fetal effects were detected at the landiolol HCl dose of 100 mg/kg/day; the resulting systemic landiolol exposure (AUC) at this dose level was not determined.
In a prenatal and postnatal development study in rats, landiolol HCl was administered intravenously at 25, 50, or 100 mg/kg/day from gestation day 17 to postpartum/lactation day 20. A decrease in the viability index for the offspring on postpartum day 4 was observed for the high dose group. No effect on pre/post-natal development was observed at 50 mg/kg dose, which represents landiolol exposures approximately 5.4 times the human exposure at the MRHD.
8.2 Lactation
Risk Summary
There are no data on the presence of landiolol and its metabolites in human milk, the effects on the breastfed infant, or the effects on milk production. However, other drugs in this class are detected in human milk.
Landiolol was present in the milk of lactating rats (see Data). When a drug is present in animal milk, it is likely that the drug will be present in human milk, but the concentration of landiolol in animal milk does not necessarily predict the concentration of drug in human milk.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for RAPIBLYK and any potential adverse effects on the breast fed infant from RAPIBLYK or from the underlying maternal condition.
Clinical Considerations
Monitoring for adverse reactions
Monitor the breastfed infant for bradycardia and other symptoms of beta blockade, such as lethargy (hypoglycemia).
Data
In a lactation study, administration of 14C-landiolol HCl administered as a single 1 mg/kg intravenous injection to lactating rats on postpartum/lactation day 13 to 14 was excreted in milk corresponding to approximately 70% of the concentration in plasma.
8.3 Pediatric Use
The safety and effectiveness of RAPIBLYK injection in pediatric patients have not been established.
8.4 Geriatric Use
Clinical studies of RAPIBLYK did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, dose selection for an elderly patient should usually start at the low end of the dosing range, reflecting greater frequency of decreased renal or cardiac function and of concomitant disease or other drug therapy.
8.5 Hepatic Impairment
More conservative dose titration is recommended in patients with mild hepatic impairment (Child-Pugh A). The effect of moderate or severe hepatic impairment (Child-Pugh B or C) on landiolol pharmacokinetics is unknown. Avoid use of RAPIBLYK in patients with moderate or severe hepatic impairment (Child-Pugh B or C). [see Pharmacokinetics (12.3)].
-
10 OVERDOSAGE
10.1 Signs and Symptoms of Overdose
Overdoses of RAPIBLYK injection can cause adverse cardiac and central nervous system effects. These adverse effects may precipitate severe signs, symptoms, sequelae, and complications such as severe cardiac and respiratory failure, including shock and coma, and may be fatal. Continuous monitoring of the patient is required.
- Cardiac adverse effects include bradycardia, atrioventricular block (1-, 2-, 3-degree), junctional rhythms, intraventricular conduction delays, decreased cardiac contractility, hypotension, cardiac failure (including cardiogenic shock), cardiac arrest/asystole, and pulseless electrical activity.
- Central nervous system adverse effects include respiratory depression, seizures, sleep and mood disturbances, fatigue, lethargy, and coma.
- In addition, bronchospasm, hyperkalemia, and hypoglycemia (especially in children) may occur.
10.2 Treatment Recommendations
Because of its approximately 4-minute elimination half-life, the first step in the management of toxicity should be to discontinue RAPIBLYK infusion. Then, based on the observed clinical effects, consider the following general measures:
Bradycardia
Consider intravenous administration of atropine or another anticholinergic drug or cardiac pacing.
Cardiac Failure
Consider intravenous administration of a diuretic or digitalis glycoside. In shock resulting from inadequate cardiac contractility, consider intravenous administration of dopamine, dobutamine, isoproterenol, milrinone or inamrinone.
Symptomatic Hypotension
Consider intravenous administration of fluids or vasopressor agents such as dopamine or norepinephrine.
Bronchospasm
Consider intravenous administration of a beta agonist or a theophylline derivative.
-
11 DESCRIPTION
RAPIBLYK (landiolol) for injection is a beta-1 adrenergic receptor blocker with a very short duration of action (elimination half-life is approximately 4 minutes). Landiolol is:
- [(4S)-2,2-dimethyl-1,3-dioxolan-4-yl]methyl 3-[4-[(2S)-2-hydroxy-3-[2-(morpholine-4-carbonylamino)ethylamino]propoxy]phenyl]propionate and has the following structure:
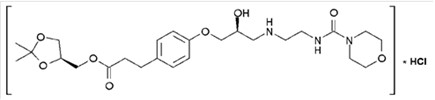
- The active pharmaceutical ingredient is the hydrochloride salt of landiolol, which has the empirical formula C 25H 39N 3O 8 ∙ HCl and a molecular weight of 546.06 g/mol. It has two chiral centers and is used as pure S,S-enantiomer.
- Landiolol HCl is a white crystalline powder. It is a relatively hydrophilic compound, which is very soluble in water.
RAPIBLYK is supplied as a single presentation of 280 mg landiolol (equivalent to 300 mg landiolol HCl) as a white to almost white sterile lyophilized powder for intravenous injection in a 50 mL vial. Inactive ingredients include 300 mg of mannitol and sodium hydroxide as needed to adjust pH.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
RAPIBLYK is a selective beta-1-adrenoreceptor antagonist that inhibits the positive chronotropic effects of the catecholamines, epinephrine and norepinephrine, on the heart, where beta-1-receptors are predominantly located. Landiolol does not exhibit any membrane-stabilizing activity or intrinsic sympathomimetic activity at the approved recommend dosage in vitro.
12.2 Pharmacodynamics
Landiolol exposure-response relationships and the time course of pharmacodynamic response are not fully characterized.
12.3 Pharmacokinetics
Landiolol peak plasma concentration (C max) over the approved dosage range in healthy volunteers and patients with atrial fibrillation or atrial flutter is provided in Table 3.
Table 3: Landiolol peak plasma concentration (C max) in healthy volunteers and patients with atrial fibrillation or atrial flutter. Landiolol Dosage
Peak Plasma Concentration (C max) Healthy Volunteers
(mean) a
Atrial Fibrillation or Atrial Flutter Patients
(range)
9.3 mcg/kg/min
0.2 mcg/mL Not studied 18.6 mcg/kg/min 0.5 mcg/mL Not studied 37.3 mcg/kg/min 1.0 mcg/mL 0.52 to 1.77 mcg/mL a 2 hour infusion
9.3 mcg/kg/min landiolol is equivalent to 10 mcg/kg/min landiolol hydrochloride.
Landiolol pharmacokinetics increases dose-proportionally in the dosage range of 9.3 to 74.6 mcg/kg/min (2 times the maximum approved recommended dosage). Landiolol reached steady-state values approximately 15 minutes after initiation of the infusion.
Distribution
Landiolol steady state volume of distribution is 0.4 L/kg. Landiolol protein binding is <10%.
Elimination
Landiolol elimination half-life is 4.5 minutes at steady state. Total body clearance of landiolol is 57 mL/kg/min following a 20-hour continuous landiolol infusion of 37.3 mcg/kg/min.
Metabolism
Landiolol is primarily metabolized by pseudocholinesterases and carboxylesterases in the plasma to the active metabolite M1.
M1 has less than 1/40 th of the pharmacological activity of landiolol. M1 AUC 0-inf is approximately 12 times greater than landiolol.
Excretion
Approximately 50 to 75% of the landiolol administered dose (approximately half of this as metabolite M1, and 8% as parent) is recovered in urine at 4 hours and 89 to 99% at 24 hours following a 60 min intravenous infusion.
Specific Populations
The effect of age and renal impairment on landiolol pharmacokinetics is unknown.
Patients with hepatic impairment
Landiolol geometric mean AUC increased by 44% and C max by 42% in 5 patients with mild hepatic impairment (Child-Pugh A) and 1 patient with moderate hepatic impairment (Child-Pugh B). The effect of moderate hepatic impairment (Child-Pugh B) on landiolol pharmacokinetics has not been fully characterized and the effect of severe hepatic impairment (Child-Pugh C) is unknown.
Drug Interaction Studies
In Vitro Studies
CYP 450 enzymes: Landiolol and the metabolite M1 are time-dependent CYP 2D6 inhibitors at exposures approximately 50 times the human exposure at the MRHD, but do not inhibit CYP 1A2, 2C9, 2C19, or 3A4.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Animal studies have not been conducted to evaluate the carcinogenic potential of RAPIBLYK.
Mutagenesis
Landiolol was not genotoxic when tested in a battery of assays (Ames, in vitro mouse lymphoma TK +/-, and in vivo micronucleus test in rats).
Impairment of Fertility
No adverse effects on fertility were observed in male and female rats administered a landiolol HCl dose of 100 mg/kg/day, resulting in systemic exposure (AUC) approximately 10-times the exposure at the MRHD
-
14 CLINICAL STUDIES
In 5 randomized, double-blind, placebo-controlled studies, treatment of 317 adults with supraventricular tachycardia with landiolol decreased heart rate in 40-90% of treated patients within about 10 minutes, compared to 0-11% of patients who received placebo; heart rate decrease was defined as a >20% decrease in heart rate or a heart rate <100 bpm or at least intermittent cessation of the arrhythmia. The infused dose of landiolol in these studies ranged from 9.3 to 74.6 mcg/kg/min (equivalent to 10 to 80 mcg/kg/min landiolol hydrochloride).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
RAPIBLYK is supplied as a white to almost white, preservative-free, lyophilized powder in single dose vials containing 280 mg landiolol for injection (equivalent to 300 mg landiolol HCl).
Each vial is supplied in a carton NDC: 84381-110-01
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
RAPIBLYK
landiolol injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 84381-110 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LANDIOLOL HYDROCHLORIDE (UNII: G8HQ634Y17) (LANDIOLOL - UNII:62NWQ924LH) LANDIOLOL 280 mg Inactive Ingredients Ingredient Name Strength SODIUM HYDROXIDE (UNII: 55X04QC32I) MANNITOL (UNII: 3OWL53L36A) 300 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 84381-110-01 1 in 1 CARTON 05/19/2025 1 1 in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA217202 05/19/2025 Labeler - AOP Orphan Pharmaceuticals GmbH (300757940)
Trademark Results [RAPIBLYK]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 RAPIBLYK 98589242 not registered Live/Pending |
AOP Orphan Pharmaceuticals GmbH 2024-06-06 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
