ENJAYMO- sutimlimab-jome injection, solution, concentrate
Enjaymo by
Drug Labeling and Warnings
Enjaymo by is a Prescription medication manufactured, distributed, or labeled by Bioverativ Therapeutics Inc., Biogen MA Inc., Vetter Pharma Fertigung GmbH & Co. KG (Ravensburg Mooswiesen), KBI Biopharma, Inc., Genzyme Corporation, Eurofins Biopharma Product Testing Munich GmbH, Sanofi-Aventis Deutschland GmbH. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ENJAYMO safely and effectively. See full prescribing information for ENJAYMO.
ENJAYMO ® (sutimlimab-jome) injection, for intravenous use
Initial U.S. Approval: 2022INDICATIONS AND USAGE
ENJAYMO is a classical complement inhibitor indicated for the treatment of hemolysis in adults with cold agglutinin disease (CAD). (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Injection: 1,100 mg/22 mL (50 mg/mL) in a single-dose vial (3)
CONTRAINDICATIONS
ENJAYMO is contraindicated in patients with known hypersensitivity to sutimlimab-jome or any of the inactive ingredients. (4)
WARNINGS AND PRECAUTIONS
- Serious Infections: Ensure patients are vaccinated against encapsulated bacteria. Monitor patients for early signs and symptoms of infections. (5.1)
- Infusion-Related Reactions: Monitor patients for infusion-related reactions, interrupt if reaction occurs, and institute appropriate medical management as needed. (5.2)
- Risk of Autoimmune Disease: Monitor patients for signs and symptoms and manage medically. (5.3)
- Recurrent Hemolysis After ENJAYMO Discontinuation: Monitor patients for signs and symptoms of hemolysis if treatment with ENJAYMO is interrupted. (5.4)
ADVERSE REACTIONS
Most common adverse reactions in the CADENZA study (Part A) (incidence ≥18%) are rhinitis, headache, hypertension, acrocyanosis, and Raynaud's phenomenon. The most common adverse reactions in the CARDINAL study (incidence ≥25%) are urinary tract infection, respiratory tract infection, bacterial infection, dizziness, fatigue, peripheral edema, arthralgia, cough, hypertension, and nausea. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Bioverativ Therapeutics Inc. (A SANOFI COMPANY) at 1-800-745-4447 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 2/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Vaccinations for Encapsulated Bacterial Infections
2.2 Recommended Dosage Regimen
2.3 Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Serious Infections Including Those Caused by Encapsulated Bacteria
5.2 Infusion-Related Reactions
5.3 Risk of Autoimmune Disease
5.4 Recurrent Hemolysis After ENJAYMO Discontinuation
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 CADENZA
14.2 CARDINAL
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Vaccinations for Encapsulated Bacterial Infections
Vaccinate patients against encapsulated bacteria, including Streptococcus pneumoniae and Neisseria meningitidis (serogroups A, C, W, Y and B), according to current Advisory Committee on Immunization Practices (ACIP) recommendations at least 2 weeks prior to initiation of ENJAYMO [see Warnings and Precautions (5.1)]. If urgent ENJAYMO therapy is indicated in a patient who is not up to date with vaccines for Streptococcus pneumoniae and Neisseria meningitidis administer these vaccines as soon as possible.
2.2 Recommended Dosage Regimen
The recommended dosage of ENJAYMO for patients with CAD is based on body weight. For patients weighing 39 kg to less than 75 kg, the recommended dose is 6,500 mg and for patients weighing 75 kg or more, the recommended dose is 7,500 mg. Administer ENJAYMO intravenously weekly for the first two weeks, with administration every two weeks thereafter. Administer ENJAYMO at the recommended dosage regimen time points, or within two days of these time points.
If a dose is missed, administer as soon as possible; thereafter, resume dosing every two weeks. If the duration after the last dose exceeds 17 days, administer ENJAYMO weekly for two weeks, with administration every two weeks thereafter.
2.3 Preparation and Administration
ENJAYMO is for intravenous infusion only.
Each vial of ENJAYMO is intended for single dose only.
ENJAYMO can either be used as an undiluted or diluted preparation.
Undiluted preparation of ENJAYMO
Use aseptic technique to prepare ENJAYMO as follows:
- Remove ENJAYMO from the refrigerator. To minimize foaming, do not shake ENJAYMO.
- Inspect vials visually for particulate matter and discoloration prior to administration. ENJAYMO solution is a clear to slightly opalescent and colorless to slightly yellow liquid. Do not administer if discolored or if other foreign particulate matter is present.
- Withdraw the calculated volume of ENJAYMO from the appropriate number of vials based on the recommended dosage (see Table 1) and add to an empty infusion bag.
- Prior to administration, allow the infusion solution to adjust to room temperature (59°F to 77°F (15°C to 25°C). Refer to Table 1 for infusion rate. The infusion should be administered over 1 hour. Administer ENJAYMO infusion solution only through a 0.2 micron in-line filter with a polyethersulfone (PES) membrane.
- The infusion catheter and tubing should be primed with the dosing solution immediately before infusion and flushed immediately following completion of the infusion with a sufficient quantity (approximately 20 mL) of sterile 0.9% Sodium Chloride Injection, USP.
- If the ENJAYMO infusion solution is not used immediately, store refrigerated at 36°F to 46°F (2°C to 8°C).
- Once removed from refrigeration, allow the ENJAYMO infusion solution to adjust to room temperature 59°F to 77°F (15°C to 25°C) and administer within 8 hours. Total time from the time of preparation, including refrigeration, adjustment to room temperature and the expected infusion time should not exceed 36 hours. In-line infusion warmers may be used, do not exceed a temperature of 104°F (40°C).
- No incompatibilities have been observed between ENJAYMO infusion solution and infusion bags made of Di-(2-ethylhexyl)phthalate (DEHP) plasticized polyvinyl chloride (PVC), Ethyl Vinyl Acetate (EVA) and polyolefin (PO); administration sets made of DEHP-plasticized PVC, DEHP-free polypropylene (PP) and polyethylene (PE); and vial adapters made of polycarbonate (PC) and acrylonitrile-butadiene-styrene (ABS).
Table 1: Infusion Reference Table for ENJAYMO (undiluted) Body Weight Range Dose Number of ENJAYMO Vials Needed ENJAYMO Volume Maximum Infusion Rate - * Patients with cardiopulmonary disease may receive the infusion over 120 minutes.
Greater than or equal to 39 kg to less than 75 kg 6,500 mg 6 130 mL 130 mL/hour* 75 kg or greater 7,500 mg 7 150 mL 150 mL/hour* Diluted preparation of ENJAYMO
Use aseptic technique to prepare ENJAYMO as follows:
- Remove ENJAYMO from the refrigerator. To minimize foaming, do not shake ENJAYMO.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- ENJAYMO solution is a clear to slightly opalescent and colorless to slightly yellow solution. Do not administer if discolored or if foreign particulate matter is present.
- Withdraw the calculated volume of ENJAYMO from the appropriate number of vials based on the recommended dosage (see Table 1). Dilute the calculated volume with 0.9% Sodium Chloride Injection, USP to a total volume of 500 mL.
- Refer to Table 2 for infusion rate. Administer the infusion over 1 to 2 hours depending on the patient's body weight. Administer ENJAYMO infusion solution only through a 0.2 micron in-line filter with a polyethersulfone (PES) membrane.
- Prime the infusion tubing with the dosing solution immediately before infusion and flush immediately following completion of the infusion with a sufficient quantity (approximately 20 mL) of 0.9% Sodium Chloride Injection, USP.
- If the ENJAYMO infusion solution is not used immediately, store refrigerated at 36°F to 46°F (2°C to 8°C).
- Once removed from refrigeration, allow the ENJAYMO infusion solution to adjust to room temperature 59°F to 77°F (15°C to 25°C) and administer within 8 hours. Total time from the time of preparation, including refrigeration, adjustment to room temperature and the expected infusion time should not exceed 36 hours. In-line infusion warmers may be used; do not exceed a temperature of 104°F (40°C).
- No incompatibilities have been observed between ENJAYMO infusion solution and infusion bags made of Di-(2-ethylhexyl)phthalate (DEHP) plasticized polyvinyl chloride (PVC), Ethyl Vinyl Acetate (EVA) and polyolefin (PO); administration sets made of DEHP-plasticized PVC, DEHP-free polypropylene (PP) and polyethylene (PE); and vial adapters made of polycarbonate (PC) and acrylonitrile-butadiene-styrene (ABS).
Table 2: Infusion Reference Table for ENJAYMO (diluted in 0.9% Sodium Chloride Injection, USP) Body Weight Range Dose Number of ENJAYMO Vials Needed ENJAYMO Volume Volume of NaCl Diluent Total Volume Maximum Infusion Rate - * Patients with cardiopulmonary disease may receive the infusion over 120 minutes.
39 kg to less than 70 kg 6,500 mg 6 130 mL 370 mL 500 mL 250 mL/hour 70 kg to less than 75 kg 6,500 mg 6 130 mL 370 mL 500 mL 500 mL/hour* 75 kg or greater 7,500 mg 7 150 mL 350 mL 500 mL 500 mL/hour* Slow or stop the infusion in case of infusion reaction during ENJAYMO administration. Monitor the patient for at least two hours following completion of the initial infusion for signs or symptoms of an infusion and/or hypersensitivity reaction. Monitor the patient for one hour following completion of subsequent infusions for signs or symptoms of an infusion reaction.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
ENJAYMO is contraindicated in patients with known hypersensitivity to sutimlimab-jome or any of the inactive ingredients [see Warnings and Precautions (5.2) and Adverse Reactions (6.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Serious Infections Including Those Caused by Encapsulated Bacteria
ENJAYMO, a proximal classical complement C1s inhibitor, increases a patient's susceptibility to serious infections including those caused by encapsulated bacteria e.g. Neisseria meningitidis (any serogroup, including non-groupable strains), Streptococcus pneumoniae, and Haemophilus influenzae type B.
Life-threatening and fatal infections with encapsulated bacteria have occurred in both vaccinated and unvaccinated patients treated with complement inhibitors.
Serious infections (bacterial and viral) were reported in 15% (10/66) of patients receiving ENJAYMO from the two phase 3 studies. These infections included urinary tract infection with sepsis, respiratory tract infection, pneumonia, otomastoiditis, and skin infections One patient (1.5%) died due to klebsiella pneumonia.
Complete or update vaccination against encapsulated bacteria at least 2 weeks prior to administration of the first dose of ENJAYMO, according to the most current ACIP recommendations for patients receiving a complement inhibitor. Revaccinate patients in accordance with ACIP recommendations considering the chronic duration of therapy with ENJAYMO. Note that, ACIP recommends an administration schedule in patients receiving complement inhibitors that differs from the administration schedule in the vaccine prescribing information. If urgent ENJAYMO therapy is indicated in a patient who is not up to date on their vaccines administer vaccine(s) as soon as possible.
Vaccination does not eliminate the risk of serious encapsulated bacterial infections, despite development of antibodies following vaccination. Closely monitor patients for early signs and symptoms of serious infection and evaluate patients immediately if an infection is suspected.
If ENJAYMO treatment is administered to patients with active systemic infections, monitor closely for signs and symptoms of worsening infection. Some infections may become rapidly life-threatening or fatal if not recognized and treated promptly. Inform patients of these signs and symptoms and steps to be taken to seek immediate medical care. Consider interruption of ENJAYMO treatment in patients who are undergoing treatment for serious infection. ENJAYMO has not been studied in patients with chronic systemic infections such as hepatitis B, hepatitis C, or HIV. Consider patients' immune status when initiating treatment with ENJAYMO.
5.2 Infusion-Related Reactions
ENJAYMO is contraindicated in patients with known hypersensitivity to sutimlimab-jome or any of the inactive ingredients [see Contraindications (4)]. Administration of ENJAYMO may result in infusion-related reactions. In the two phase 3 studies, 19 of 66 (29%) patients treated with ENJAYMO experienced infusion-related reactions (e.g., shortness of breath, rapid heartbeat, nausea, flushing, headache, hypotension, chest discomfort, pruritus, rash, injection site reaction, and dizziness) were reported in patients from the two clinical studies. One patient permanently discontinued ENJAYMO due to an infusion-related reaction.
Monitor patients for infusion-related reactions and interrupt if a reaction occurs. Discontinue ENJAYMO infusion and institute appropriate supportive measures if signs of hypersensitivity reactions, such as cardiovascular instability or respiratory compromise, occur.
5.3 Risk of Autoimmune Disease
Based on its mechanism of action, ENJAYMO may potentially increase the risk for developing autoimmune diseases such as systemic lupus erythematosus (SLE). Development of systemic lupus erythematosus (SLE) has been associated with inherited classical complement deficiency. Patients with SLE or autoimmune disease with positive anti-nuclear antibody were excluded from clinical trials with ENJAYMO. In clinical trials, 3/66 (4.5%) patients developed a relapse or worsening of preexisting autoimmune disease. Monitor patients being treated with ENJAYMO for signs and symptoms and manage medically.
5.4 Recurrent Hemolysis After ENJAYMO Discontinuation
If treatment with ENJAYMO is interrupted, closely monitor patients for signs and symptoms of recurrent hemolysis, e.g., elevated levels of total bilirubin or lactate dehydrogenase (LDH) accompanied by a decrease in hemoglobin, or reappearance of symptoms such as fatigue, dyspnea, palpitations, or hemoglobinuria. Consider restarting ENJAYMO if signs and symptoms of hemolysis occur after discontinuation.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed in greater detail in other sections of the labeling:
- Serious Infections [see Warnings and Precautions (5.1)]
- Infusion-Related Reactions [see Warnings and Precautions (5.2)]
- Risk of Autoimmune Disease [see Warnings and Precautions (5.3)]
- Recurrent Hemolysis After ENJAYMO Discontinuation [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of ENJAYMO in patients with a confirmed diagnosis of CAD was evaluated in a placebo-controlled study (CADENZA) in Part A (n=42) followed by an open-label single-arm study in Part B (n=39) and an open-label single-arm study (CARDINAL) (n=24) [see Clinical Studies (14)]. The median duration of treatment exposure to ENJAYMO was 104 weeks (patients randomized to ENJAYMO in CADENZA Part A) and 93 weeks (patients randomized to placebo in CADENZA Part A) and 143 weeks for CARDINAL.
CADENZA (Part A)
Serious adverse reaction occurred in 2/22 (9%) patients who received ENJAYMO. Serious adverse reactions included Raynaud's phenomenon (n=1) and febrile infection (n=1).
Permanent discontinuation of ENJAYMO due to an adverse reaction occurred in 2/22 (9%) patients. Adverse reactions which resulted in permanent discontinuation of ENJAYMO included Raynaud's phenomenon (n=1), acrocyanosis (n=1), and infusion related reactions (n=1).
Dosage interruptions of ENJAYMO due to an adverse reaction occurred in 3/22 patients. Adverse reactions which required dosage interruption included nasopharyngitis (n=1) and infusion related reaction (n=1), including pruritis (n=1) and chest discomfort (n=1).
The most common adverse reactions (≥18%) reported in the CADENZA study were rhinitis, headache, hypertension, acrocyanosis, and Raynaud's phenomenon.
Table 3: Adverse Reactions (≥10%) in Patients Who Received ENJAYMO with a Difference Between Arms of >5% Compared to Placebo in the CADENZA Study (Part A) Adverse Reactions ENJAYMO
(N=22)Placebo
(N=20)Headache 5 (23%) 2 (10%) Hypertension 5 (23%) 0 Rhinitis 4 (18%) 0 Acrocyanosis 4 (18%) 0 Raynaud's phenomenon 4 (18%) 0 CARDINAL
Serious adverse reactions occurred in 10/24 (42%) patients who received ENJAYMO. The most common adverse reaction (>5%) was acrocyanosis (n=2). A fatal adverse reaction of pneumonia klebsiella occurred in one patient who received ENJAYMO.
Permanent discontinuation of ENJAYMO due to an adverse reaction occurred in 2/24 (8%) patients. Adverse reactions which resulted in permanent discontinuation of ENJAYMO included pneumonia klebsiella (n=1) and acrocyanosis (n=2).
Dosage interruptions of ENJAYMO due to an adverse reaction occurred in 7/24 patients. Adverse reactions which required dosage interruption included pneumonia, COVID-19 pneumonia, abdominal pain upper, urinary tract infection bacterial, urosepsis, acrocyanosis, viral infection, blood creatinine increased and infusion-related reaction.
The most common adverse reaction (≥25%) reported in the CARDINAL study were urinary tract infection, respiratory tract infection, bacterial infection, dizziness, fatigue, peripheral edema, arthralgia, cough, hypertension, and nausea.
Table 4: Adverse Reactions (≥15%) in Patients Receiving ENJAYMO in the CARDINAL Study Adverse Reaction/Body System n (%)
(N=24)Please note: if a subject has multiple events in a grouped term the subject is only counted once. The following terms were combined for the analysis: - * Urinary Tract Infection includes cystitis, urosepsis
- † Respiratory tract infection includes upper respiratory tract infection, bronchitis, lower respiratory tract infection, COVID-19 pneumonia
- ‡ Bacterial infection includes Escherichia urinary tract infection, urinary tract infection bacteria, cystitis bacterial, Escherichia sepsis, pneumococcal sepsis, pneumonia klebsiella, streptococcal sepsis, wound infection staphylococcal
- § Viral infection includes oral herpes, herpes zoster, respiratory tract infection viral, viral upper respiratory tract infection, Herpes simplex viraemia
- ¶ Dizziness includes dizziness postural and vertigo
- # Fatigue includes asthenia, malaise, mental fatigue
- Þ Peripheral edema includes peripheral swelling
- ß Hypertension includes, blood pressure increased, essential hypertension
- à Abdominal pain includes abdominal pain upper, abdominal tenderness
- è Cough includes productive cough
- ð Infusion related reaction includes stress cardiomyopathy, feeling cold (All occurred within 24 hours of start of ENJAYMO infusion)
INFECTIONS AND INFESTATIONS Urinary tract infection* 9 (38%) Respiratory tract infection† 6 (25%) Bacterial infection‡ 6 (25%) Nasopharyngitis 5 (21%) Viral infection§ 5 (21%) NERVOUS SYSTEM DISORDERS Dizziness¶ 7 (29%) Headache 5 (21%) GENERAL DISORDERS Fatigue# 8 (33%) Peripheral edemaÞ 6 (25%) Pyrexia 5 (21%) MUSCULOSKELETAL AND CONNECTIVE TISSUE DISORDERS Arthralgia 6 (25%) VASCULAR DISORDERS Hypertensionß 6 (25%) Acrocyanosis 5 (21%) GASTROINTESTINAL DISORDERS Nausea 6 (25%) Abdominal painà 5 (21%) RESPIRATORY, THORACIC, AND MEDIASTINAL DISORDERS Coughè 6 (25%) INJURY, POISONING AND PROCEDURAL COMPLICATIONS Infusion-related reactionð 4 (17%) -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on ENJAYMO use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Human immunoglobulin G (IgG) antibodies are known to cross the placental barrier; therefore, sutimlimab-jome may be transmitted from the mother to the developing fetus. In animal reproduction studies, intravenous administration of sutimlimab-jome to pregnant monkeys during organogenesis at doses 2 to 3 times the maximum recommended human doses did not result in adverse effects on pregnancy or offspring development (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2%–4% and 15%–20%, respectively.
Data
Animal data
Pregnant monkeys were administered sutimlimab-jome at doses of 60 and 180 mg/kg/dose via 30-minute intravenous infusion once-weekly from gestation Day 20 to parturition (approximately 21 doses) resulting in exposures 2 to 3 times the human exposures at the maximum recommended doses, based on area under the curve (AUC). Sutimlimab-jome was detectable in infants born to pregnant females exposed to 180 mg/kg/week. No effects on reproductive and developmental parameters were observed in maternal animals and offspring, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of sutimlimab-jome in human milk, effects on the breastfed child, or the effects on milk production. Maternal IgG is known to be present in human milk. The effects of local gastrointestinal exposure and limited systemic exposure in the breastfed child to sutimlimab-jome are unknown. No conclusions can be drawn regarding whether or not ENJAYMO is safe for use during breastfeeding. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for ENJAYMO and any potential adverse effects on the breastfed child from ENJAYMO or from the underlying maternal condition.
8.5 Geriatric Use
Of the 66 patients with CAD in clinical studies of ENJAYMO, 65% were 65 years of age and over, including 27% who were 75 years of age and over. No overall differences in safety or effectiveness were observed between these patients and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
-
11 DESCRIPTION
Sutimlimab-jome, a classical complement inhibitor, is a humanized monoclonal antibody expressed by recombinant in Chinese hamster ovary (CHO) cells and produced in vitro using standard mammalian cell culture methods. Sutimlimab-jome is composed of two heterodimers. Each heterodimer is composed of a heavy and a light polypeptide chain. Each heavy chain (H-chain) is composed of 445 amino acids and each light chain (L-chain) contains 216 amino acids. Sutimlimab-jome has a molecular weight of approximately 147 kDa.
ENJAYMO (sutimlimab-jome) injection is a sterile, clear to slightly opalescent, colorless to slightly yellow, preservative-free solution for intravenous use. Each single-dose vial contains 1,100 mg sutimlimab-jome at a concentration of 50 mg/mL with a pH of 6.1. Each mL contains 50 mg of sutimlimab-jome and also contains polysorbate 80 (0.2 mg), sodium chloride (8.18 mg), sodium phosphate dibasic heptahydrate (0.48 mg), sodium phosphate monobasic monohydrate (1.13 mg), and Water for Injection, USP.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Sutimlimab-jome is an immunoglobulin G (IgG), subclass 4 (IgG4) monoclonal antibody (mAb) that inhibits the classical complement pathway (CP) and specifically binds to complement protein component 1, s subcomponent (C1s), a serine protease which cleaves C4. Sutimlimab-jome does not inhibit the lectin and alternative pathways. Inhibition of the classical complement pathway at the level of C1s prevents deposition of complement opsonins on the surface of RBCs, resulting in inhibition of hemolysis in patients with CAD.
12.2 Pharmacodynamics
Greater than 90% inhibition of CP was observed following a single sutimlimab-jome infusion and sustained in patients with CAD when sutimlimab-jome concentrations were greater than or equal to 100 mcg/mL. C4 levels returned to normal levels (0.2 g/L) in patients with CAD within one week following the first dose of sutimlimab-jome. Complete CP inhibition following initiation of sutimlimab-jome treatment led to inhibition of hemolysis as evidenced by normalization of bilirubin, decrease in LDH, increase in haptoglobin, and decrease in reticulocytes.
After the first treatment with sutimlimab-jome, near normalization of bilirubin associated with a greater than 1 g/dL increase in hemoglobin was observed, demonstrating the effect of CP inhibition. The extent and duration of the pharmacodynamic response in patients with CAD were exposure dependent for sutimlimab-jome.
12.3 Pharmacokinetics
Following administration of the approved weight-based recommended dosages, the exposure of sutimlimab-jome increases proportionally over a dosage range of 60 mg/kg to 100 mg/kg by intravenous infusion (0.3 to 1.5 times the maximum approved recommended dosage based on 75 kg body weight). Steady state was achieved by Week 7 after starting sutimlimab-jome treatment, with an accumulation ratio of less than 2.
Distribution
Sutimlimab-jome binds to C1s in the serum. The volume of distribution at steady state was approximately 5.8 L in patients with CAD.
Elimination
The terminal elimination half-life and clearance varies at different doses due to target-mediated drug disposition at lower sutimlimab-jome concentrations. The terminal elimination half-life (t1/2β) of sutimlimab-jome is 21 days with a clearance (CL) of approximately 0.14 L/day at the approved recommended dosage.
Specific Populations
No clinically significant differences in the pharmacokinetics of sutimlimab-jome were observed based on sex, age (19 to 88 years of age), ethnicity (Japanese, non-Japanese), and mild to moderate renal impairment (30 to 89 mL/min/1.73 m2 measured by estimated glomerular filtration rate [eGFR]). The effects of severe renal impairment and hepatic impairment on the pharmacokinetics of sutimlimab-jome are unknown.
Body weight
Population pharmacokinetic analysis shows that sutimlimab-jome AUC at steady-state decreased up to 40% for a patient weighing 110 kg following the 7.5 g dose and increased up to 170% for a patient weighing 40 kg following the 6.5 g dose as compared with a patient weighing 70 kg following the 6.5 g dose. The effect of body weight on pharmacokinetics has been integrated in the recommended dose regimen tiered by body weight.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of sutimlimab-jome or of other sutimlimab products.
During the treatment period in CARDINAL and CADENZA, 8/66 (12%) ENJAYMO-treated patients developed anti-sutimlimab-jome antibodies (duration of exposure up to 177 weeks). There was no identified clinically significant effect of anti-drug antibodies on pharmacokinetics, pharmacodynamics, safety, or effectiveness of ENJAYMO over the treatment duration [see Clinical Studies (14)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity and mutagenicity studies have not been conducted with sutimlimab-jome.
Effects of sutimlimab-jome on male and female fertility have not been studied in animals. In repeat-dose studies in cynomolgus monkeys with sutimlimab-jome administered once-weekly at exposures 3 to 4 times the human exposures at the maximum recommended human doses of sutimlimab-jome, no effects on male or female reproductive tissues were observed.
-
14 CLINICAL STUDIES
14.1 CADENZA
The efficacy of ENJAYMO was assessed in a placebo-controlled 6-month trial in 42 patients (CADENZA, NCT 03275454). Following the completion of the 6-month treatment period (Part A) in which 22 patients received ENJAYMO and 20 patients received placebo, 39 patients (19 patients on ENJAYMO and 20 patients on placebo) continued to receive ENJAYMO in a long-term safety and durability of response extension phase (Part B) for an additional 12 months following last patient out from Part A. The trial included a 9 week safety follow-up after the last dose of ENJAYMO. Patients with a confirmed diagnosis of CAD based on chronic hemolysis, polyspecific direct antiglobulin test (DAT), monospecific DAT specific for C3d, cold agglutinin titer ≥64 at 4°C, an IgG DAT ≤1+ and no history of transfusion within 6 months, or more than one blood transfusion in the 12 months prior to enrollment in the trial were administered 6.5 g or 7.5 g ENJAYMO (based on body weight) intravenously over approximately 60 minutes on Day 0, Day 7, and every 14 days thereafter; or placebo. Patients with cold agglutinin disease secondary to infection, rheumatologic disease, systemic lupus erythematosus, or overt hematologic malignancy were excluded, whereas patients with a history of or concomitant low-grade lymphoproliferative disease were not excluded.
Major baseline characteristics of the study population are summarized in Table 5.
Table 5: Baseline Characteristics of Patients Included in CADENZA Parameter Statistic CADENZA Placebo ENJAYMO N=20 N=22 - * Placebo N=18 and ENJAYMO N= 20 in CADENZA, for bilirubin data excluding patients with either a positive or no available test result for Gilbert's syndrome.
- † ULN: Upper limit of normal, FACIT: Functional Assessment of Chronic Illness Therapy (FACIT-Fatigue is measured on a scale of 0 (worst fatigue) to 52 (no fatigue)
Age Mean 68.2 65.3 Min, Max 51, 83 46, 88 Sex Male n (%) 4 (20.0) 5 (22.7) Female 16 (80.0) 17 (77.3) Body weight Mean, Kg 64.9 66.8 Min, Max 48, 95 39, 100 Hemoglobin Mean, g/dL 9.33 9.15 Bilirubin (total)* µmol/L 35.77 41.17 (1.75 × ULN) (2 × ULN) LDH U/L 380.8 421.5 History of transfusion Mean number of transfusions (range) Within last 6 months 0 0 Within last 12 months 0 0.14 (0, 1) FACIT†-Fatigue scale Mean 32.99 31.67 Efficacy was based on the proportion of patients who met the following criteria: an increase from baseline in Hgb level ≥1.5 g/dL at the treatment assessment time point (mean value from Weeks 23, 25, and 26), no blood transfusion from Week 5 through Week 26, and no treatment for CAD beyond what was permitted per protocol from Week 5 through Week 26. Efficacy was further assessed based on the effect of ENJAYMO on Hgb, laboratory measures of hemolysis including mean change from baseline in total bilirubin and LDH. Supportive efficacy data collected included transfusion usage after five weeks of treatment. In addition, mean change from baseline in symptoms and impacts of fatigue were assessed using a patient-reported outcome instrument, the FACIT-Fatigue (score range from 0 to 52 with higher scores indicating less fatigue).
The data from this study demonstrated a statistically significant treatment effect of ENJAYMO over placebo in terms of the rate of patients who met the efficacy criteria (responder) as well as improving symptoms and impacts of fatigue (FACIT-Fatigue). The responder rate difference between ENJAYMO and placebo was 58.78% (95% CI: 34.6% to 82.96%) with a p-value of 0.0004. At the treatment assessment timepoint (TAT), 16 of 22 patients on ENJAYMO (72.7%; 95% CI: 49.8% to 89.3%) and 3 of 20 patients on placebo (15.0%; 95% CI: 3.2% to 37.9%) met primary criteria. Efficacy of ENJAYMO in the inhibition of hemolysis in patients with CAD was demonstrated across multiple end points as described in the table below (see Table 6).
Table 6: Efficacy Results in Patients with CAD in the CADENZA Part A Study Parameter Statistic Placebo
N=20ENJAYMO
N=22Treatment Effect - * A responder was defined as a patient with an increase from baseline in Hgb level ≥ 1.5 g/dL at the treatment assessment time point (mean value from Weeks 23, 25, and 26), no blood transfusion from Week 5 through Week 26, and no treatment for CAD beyond what was permitted per protocol from Week 5 through Week 26.
- † The Mantel-Haenszel stratum-weighted estimator of the rate difference with 95% CI was calculated using the Sato variance estimator. The stratification factors are baseline hemoglobin (< median vs ≥ median) and geographic region (Asia/Other, North America, and Europe)
- ‡ LS: Least Square, FACIT-Fatigue: Functional Assessment of Chronic Illness Therapy-Fatigue Scale NC= Not calculated
- § Prohibited therapies included rituximab alone or in combination with cytotoxic agents
Responder* n (%) 3 (15) 16 (72.7) 58.78 (34.6, 82.96)† p-value: <0.001 Hemoglobin Mean change from baseline (LS‡ Mean), g/dL 0.09 2.66 2.56 95% CI of LS Mean (1.75, 3.38) p-value: <0.001 Patients with mean change from baseline of:
greater than or equal to 1.5 g/dLn (%) 3 (15) 16 (72.7) NC Patients not receiving blood transfusion from Week 5 through Week 26 (transfusion avoidance) n (%) 16 (80) 18 (81.8) NC Patients not receiving protocol-prohibited CAD medications from Week 5 through Week 26§ n (%) 20 (100) 19 (86.4) NC FACIT‡-Fatigue Mean change from baseline (LS‡ Mean) 1.91 10.83 8.93 95% CI of LS Mean (4, 13.85) p-value: <0.001 During Part A, an increase in mean hemoglobin level of 2.02 g/dL was observed in patients on ENJAYMO at Week 3; in the placebo group the mean hemoglobin level decreased by 0.31g/dL. At treatment assessment timepoint, a mean decrease in bilirubin of 1.29 mg/dL compared to baseline was reported in patients on ENJAYMO (n=17) versus 0.11 mg/dL on placebo (n=18). In the ENJAYMO group, bilirubin levels normalized in 88.2% (n=15) of patients compared to 22.2% (n=4) of patients in the placebo arm. At treatment assessment timepoint, a mean decrease in LDH of 150.83 U/L compared to baseline was reported in patients on ENJAYMO (n=19) versus an increase of 7.6 U/L on placebo (n=20). In the ENJAYMO group, LDH levels were < 1.5 × ULN in 94.7% (n=18) of patients compared to 70% (n=14) in the placebo arm.
In Part B, mean hemoglobin levels were maintained at >10.5 g/dL. Sustained normalization of mean bilirubin levels was also observed indicating a sustained decrease in hemolysis. Mean hemoglobin level of 11.58 g/dL (range: 6.90–15.30) and 1.01 mg/dL (range: 0.29–5.54) for bilirubin was observed at the last on-treatment visit.
After the last dose of ENJAYMO in the study, signs and symptoms of recurrent hemolysis were observed, nine weeks after the last dose in Part B; mean hemoglobin decreased by 2.41 g/dL (SE: 0.373) and mean bilirubin increased by 1.27 mg/dL (SE: 0.182) from the last available values during treatment.
14.2 CARDINAL
The efficacy of ENJAYMO was assessed in an open-label, single-arm, 6-month trial in 24 patients (CARDINAL, NCT03347396). Following the completion of the 6-month treatment period (Part A), patients continued to receive ENJAYMO in a long-term safety and durability of response extension phase (Part B) for an additional 24 months following last patient out from Part A. The trial included a 9 week safety follow-up after the last dose of ENJAYMO.
Patients with a confirmed diagnosis of CAD based on chronic hemolysis, polyspecific direct antiglobulin test (DAT), monospecific DAT specific for C3d, cold agglutinin titer ≥64 at 4°C, and IgG DAT ≤1+ and a recent blood transfusion in the 6 months prior to enrollment were administered 6.5 g or 7.5 g ENJAYMO (based on body weight) intravenously over approximately 60 minutes on Day 0, Day 7, and every 14 days thereafter. Patients with cold agglutinin syndrome secondary to infection, rheumatologic disease, systemic lupus erythematosus, or overt hematologic malignancy were excluded, whereas patients with a history of or concomitant low-grade lymphoproliferative disease were not excluded. Major baseline characteristics of the trial population are summarized in Table 7.
Table 7: Baseline Characteristics of Patients Included in CARDINAL Parameter Statistic ENJAYMO
N=24- * N=21 for bilirubin data excluding patients with Gilbert's syndrome.
- † ULN: Upper limit of normal, LDH: Lactate dehydrogenase.
Age Mean (SD)
Range71.3 (8.2)
55 to 85 yearsSex Female n (%) 15 (63) Male 9 (38) Body weight Mean (SD)
Range67.8 (15.8)
40 to 112 kgHemoglobin Mean (SD), g/dL 8.6 (1.16) Bilirubin (total)* Mean (SD), mg/dL 3.1 (1.41)
(2.6 × ULN†)LDH† Mean (SD), U/L 438 (484.60) Blood transfusion Median number of transfusions (range) Within last 6 months 2.0 (1, 19) Within last 12 months 2.0 (1, 23) Efficacy was based on the proportion of patients who met the following criteria: an increase from baseline in Hgb level ≥2 g/dL or a Hgb level ≥12 g/dL at the treatment assessment time point (mean value from Weeks 23, 25, and 26), no blood transfusion from Week 5 through Week 26, and no treatment for CAD beyond what was permitted per protocol from Week 5 through Week 26.
Efficacy of ENJAYMO in patients with CAD is described in Table 8.
Table 8: Efficacy Results in Patients with CAD in CARDINAL Part A Study Parameter Statistic ENJAYMO
N=24- * A responder was defined as a patient with an increase from baseline in Hgb level ≥2 g/dL or a Hgb level ≥12 g/dL at the treatment assessment time point (mean value from Weeks 23, 25, and 26), no blood transfusion from Week 5 through Week 26, and no treatment for CAD beyond what was permitted per protocol from Week 5 through Week 26.
- † Prohibited therapies included rituximab alone or in combination with cytotoxic agents.
Responder* n (%) 13 (54) Hemoglobin level ≥12 g/dL or
Increase in Hemoglobin level of ≥2 g/dLn (%) 15 (63) Hemoglobin level ≥12 g/dL n (%) 9 (38) Increase in Hemoglobin level of ≥2 g/dL n (%) 15 (63) Patients not receiving RBC transfusion from Week 5 through Week 26 (transfusion avoidance) n (%) 17 (71) Patients not receiving protocol-prohibited CAD medications† from Week 5 through Week 26 n (%) 22 (92) In Part A, among 14 patients with baseline and follow-up bilirubin values, the mean was 3.23 mg/dL (2.7-fold ULN) at baseline and 0.91 mg/dL (0.8-fold ULN) at the treatment assessment time point. The least-squares (LS) mean change was reduction of -2.23 mg/dL (95% CI: -2.49 to -1.98). Among 17 patients with baseline and follow-up LDH values, the mean LDH was 424 U/L (1.7-fold ULN) at baseline and 301 U/L (1.2-fold ULN) at the follow-up time point. The least squared mean change in LDH at the treatment assessment time point was reduction of -126 (95% CI: -218 to -35).
In CARDINAL, an increase in mean hemoglobin level of 2.29 g/dL (SE: 0.308) was observed at Week 3 and 3.18 g/dL (SE: 0.476) at treatment assessment time point. The observed model mean change in hemoglobin level from baseline at treatment assessment time point was an improvement of 2.60 g/dL (95% CI: 0.74, 4.46).
In Part B, mean hemoglobin levels were maintained at >10 g/dL. Sustained normalization of mean bilirubin levels was also observed indicating a sustained decrease in hemolysis. Mean hemoglobin level of 12.23 g/dL (range: 9.20–14.40) and 0.96 mg/dL (range: 0.4–1.7) for bilirubin was observed at the last on-treatment visit.
After the last dose of ENJAYMO in the study, signs and symptoms of recurrent hemolysis were observed, nine weeks after the last dose in Part B; mean hemoglobin decreased by 2.28 g/dL (SE: 0.402) and mean bilirubin increased by 1.42 mg/dL (SE: 0.192) from the last available values during treatment.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
ENJAYMO (sutimlimab-jome) injection is a clear to slightly opalescent, colorless to slightly yellow, preservative-free solution supplied as one 1,100 mg/22 mL (50 mg/mL) single-dose vial per carton (NDC: 80203-347-01).
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Serious Infections Including Those Caused by Encapsulated Bacteria
Advise patients of the risk of serious infection. Inform patients of the need to complete or update their vaccinations against encapsulated bacteria at least 2 weeks prior to receiving the first dose of ENJAYMO. If urgent ENJAYMO therapy is indicated in a patient who is not up to date on their vaccines administer vaccine(s) as soon as possible. Inform the patient that they are required to be revaccinated according to current ACIP recommendations for encapsulated bacteria while on ENJAYMO therapy [see Warnings and Precautions (5.1)].
Inform patients that vaccination may not prevent serious infection and strongly advise the patient to seek immediate medical attention if signs or symptoms of serious infection occur. These signs and symptoms include the following:
- fever with or without shivers or the chills
- fever and a rash
- fever with chest pain and cough
- fever with breathlessness/fast breathing
- fever with high heart rate
- headache with nausea or vomiting
- headache and a fever
- headache with a stiff neck or stiff back
- confusion
- body aches with flu-like symptoms
- clammy skin
- eyes sensitive to light
Infusion-Related Reactions
Advise patients that administration of ENJAYMO may result in infusion-related reactions including hypersensitivity reactions. Hypersensitivity reactions may be serious or life-threatening (e.g., anaphylaxis). Educate patients on the symptoms of infusion-related reactions and advise them to seek medical attention if any new symptoms of infusion-related reactions occur [see Contraindications (4) and Warnings and Precautions (5.2)].
Risk of Autoimmune Disease
Educate patients that there may be an increased risk of developing an autoimmune disease such as SLE during ENJAYMO therapy. Advise patients on signs and symptoms of SLE and to report any new symptoms of SLE and seek medical attention [see Warnings and Precautions (5.3)].
Discontinuation
Inform patients with CAD that they may develop hemolysis due to CAD when ENJAYMO is discontinued and that they should be monitored by their healthcare provider following ENJAYMO discontinuation [see Warnings and Precautions (5.4)].
This product's labeling may have been updated. For the most recent prescribing information, please visit www.enjaymo.com.
- SPL UNCLASSIFIED SECTION
-
MEDICATION GUIDE
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised:02/2024 MEDICATION GUIDE
ENJAYMO® (en-jaye-moe)
(sutimlimab-jome)
injection, for intravenous useWhat is the most important information I should know about ENJAYMO?
ENJAYMO is a medicine that affects your immune system. ENJAYMO may lower the ability of your immune system to fight infections.-
ENJAYMO increases your chance of getting serious infections including those caused by encapsulated bacteria, including Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae type B. These serious infections may quickly become life-threatening or cause death if not recognized and treated early.
- You must complete or be up to date with the vaccines against Streptococcus pneumoniae and Neisseria meningitidis at least 2 weeks before your first dose of ENJAYMO.
- If your healthcare provider decides that urgent treatment with ENJAYMO is needed, you should receive vaccinations as soon as possible.
- If you have been vaccinated against these bacteria in the past, you might need additional vaccines before starting ENJAYMO. Your healthcare provider will decide if you need additional vaccines.
- Vaccines do not prevent all infections caused by encapsulated bacteria. Call your healthcare provider or get emergency medical care right away if you get any of these signs and symptoms of a serious infection:
- fever with or without shivers or chills
- fever with chest pain and cough
- fever with high heart rate
- headache and fever
- confusion
- clammy skin
- fever and a rash
- fever with breathlessness or fast breathing
- headache with nausea or vomiting
- headache with stiff neck or stiff back
- body aches with flu-like symptoms
- eyes sensitive to light
For more information about side effects, see "What are the possible side effects of ENJAYMO?" What is ENJAYMO? ENJAYMO is a prescription medicine used to treat the breakdown of red blood cells (hemolysis) in adults with cold agglutinin disease (CAD).
It is not known if ENJAYMO is safe and effective in children.Who should not receive ENJAYMO? Do not receive ENJAYMO if you are allergic to sutimlimab-jome or any of the ingredients in ENJAYMO. See the end of this Medication Guide for a complete list of ingredients in ENJAYMO. Before receiving ENJAYMO, tell your healthcare provider about all of your medical conditions, including if you: - have a fever or infection, including a history of human immunodeficiency virus (HIV), hepatitis B, or hepatitis C.
- have an autoimmune disease such as systemic lupus erythematosus (SLE), also known as lupus.
- are pregnant or plan to become pregnant. It is not known if ENJAYMO will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if ENJAYMO passes into your breast milk. You should talk to your healthcare provider about the best way to feed your baby during treatment with ENJAYMO.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.How will I receive ENJAYMO? - ENJAYMO is given through a vein by intravenous (I.V.) infusion, usually over 1 to 2 hours.
- You will usually receive a starting dose of ENJAYMO, followed by a second dose of ENJAYMO 1 week later. Then 2 weeks after your second dose, you will start to receive an ENJAYMO infusion every 2 weeks.
- After your first infusion, you should be monitored for infusion and allergic reactions for at least 2 hours. For all future infusions, you should be monitored for infusion reactions for 1 hour. See "What are the possible side effects of ENJAYMO?"
- If you have CAD and you stop receiving ENJAYMO, your healthcare provider should monitor you closely for return of your symptoms after you stop ENJAYMO. Stopping ENJAYMO may cause the breakdown of your red blood cells due to CAD to return. Symptoms or problems that can happen due to red blood cell breakdown include:
- tiredness
- shortness of breath
- rapid heart rate
- blood in your urine or dark urine
- If you miss an ENJAYMO infusion, call your healthcare provider right away.
What are the possible side effects of ENJAYMO?
ENJAYMO can cause serious side effects, including:- See "What is the most important information I should know about ENJAYMO?"
- Infusion-related reactions. Treatment with ENJAYMO may cause infusion-related reactions, including allergic reactions that may be serious or life-threatening. Your healthcare provider may slow down or stop your ENJAYMO infusion if you have an infusion-related reaction and will treat your symptoms if needed. Tell your healthcare provider right away if you develop symptoms during your ENJAYMO infusion that may mean you are having an infusion-related reaction, including:
- shortness of breath
- decrease in blood pressure
- chest discomfort
- rapid heartbeat
- nausea
- injection site reaction
- flushing
- headache
- dizziness
- rash
- itchy skin
-
Risk of autoimmune disease. ENJAYMO may increase your risk for developing an autoimmune disease such as SLE. Tell your healthcare provider and get medical help if you develop any symptoms of SLE, including:
- joint pain or swelling
- rash on the cheeks and nose
- unexplained fever
The most common side effects of ENJAYMO include: - increase in blood pressure
- urinary tract infection
- respiratory tract infection
- bacterial infection
- swelling in lower legs or hands
- joint pain
- headache
- nausea
- runny nose
- bluish color to the lips and skin
- dizziness
- feeling tired or weak
- cough
- changes in color or sensation in the fingers and toes (Raynaud's phenomenon)
These are not all the possible side effects of ENJAYMO.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.General information about the safe and effective use of ENJAYMO. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. You can ask your pharmacist or healthcare provider for information about ENJAYMO that is written for health professionals. What are the ingredients in ENJAYMO? Active ingredient: sutimlimab-jome Inactive ingredients: polysorbate 80, sodium chloride, sodium phosphate dibasic heptahydrate, sodium phosphate monobasic monohydrate, and Water for Injection, USP. Bioverativ Therapeutics Inc., Waltham, MA 02451. A SANOFI COMPANY. US License No. 2078
Distributed by: Bioverativ U.S. LLC, Waltham, MA 02451
For more information, go to www.ENJAYMO.com or call 1-800-745-4447.
©2024 Bioverativ Therapeutics Inc. All rights reserved. -
ENJAYMO increases your chance of getting serious infections including those caused by encapsulated bacteria, including Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae type B. These serious infections may quickly become life-threatening or cause death if not recognized and treated early.
-
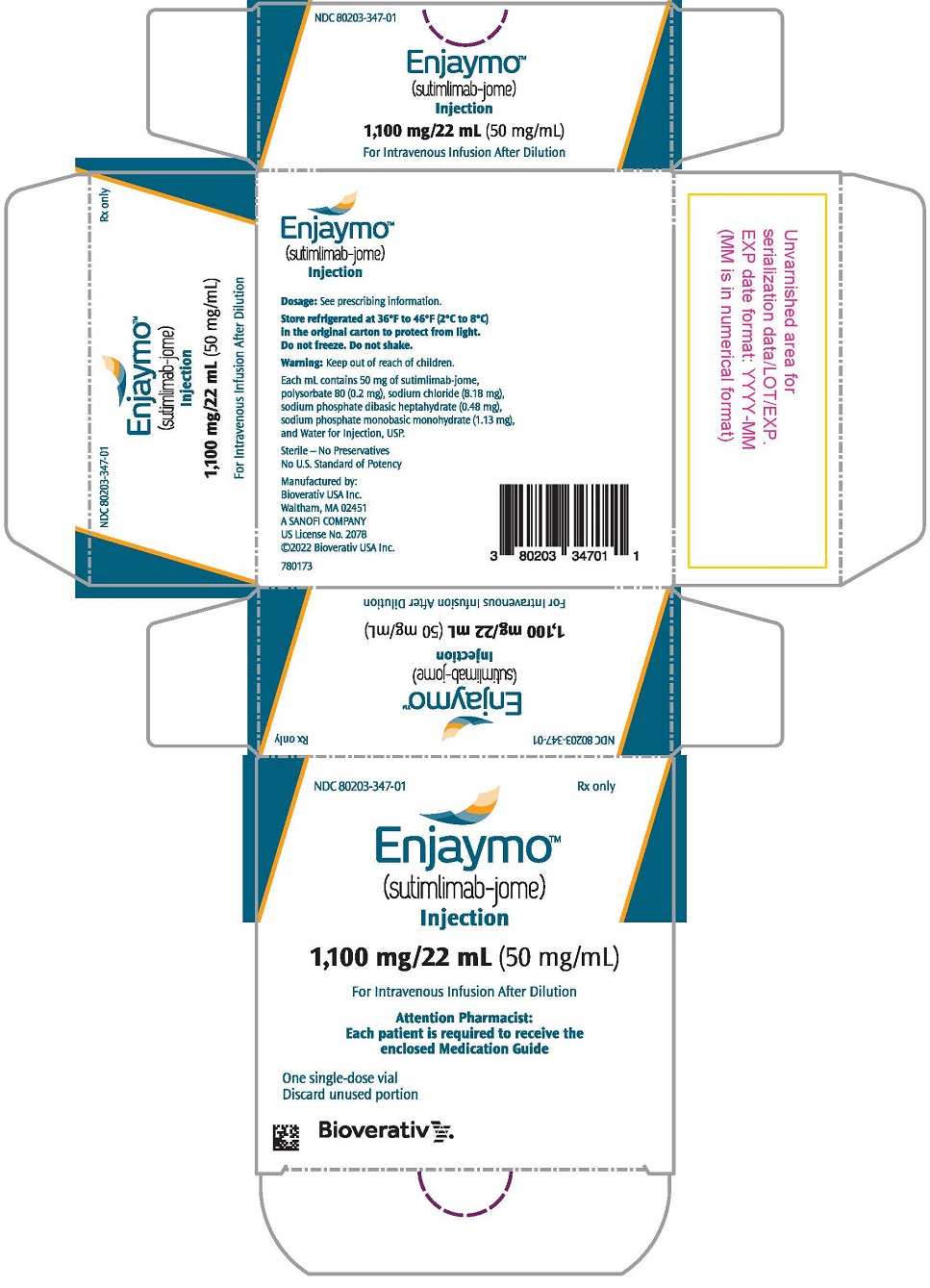
PRINCIPAL DISPLAY PANEL - 1,100 mg/22 mL Vial Carton
NDC: 80203-347-01
Rx onlyEnjaymo®
(sutimlimab-jome)
Injection1,100 mg/22 mL (50 mg/mL)
For Intravenous Infusion
Attention Pharmacist:
Each patient is required to receive the
enclosed Medication GuideOne single-dose vial
Discard unused portionsanofi
-
INGREDIENTS AND APPEARANCE
ENJAYMO
sutimlimab-jome injection, solution, concentrateProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 80203-347 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SUTIMLIMAB (UNII: GNWE7KJ995) (SUTIMLIMAB - UNII:GNWE7KJ995) SUTIMLIMAB 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.2 mg in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 8.18 mg in 1 mL SODIUM PHOSPHATE, DIBASIC, HEPTAHYDRATE (UNII: 70WT22SF4B) 0.48 mg in 1 mL SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) 1.13 mg in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 80203-347-01 1 in 1 CARTON 02/04/2022 1 22 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761164 02/04/2022 Labeler - Bioverativ Therapeutics Inc. (080521844) Establishment Name Address ID/FEI Business Operations Biogen MA Inc. 841087823 analysis(80203-347) , api manufacture(80203-347) Establishment Name Address ID/FEI Business Operations Vetter Pharma Fertigung GmbH & Co. KG (Ravensburg Mooswiesen) 312670654 analysis(80203-347) , manufacture(80203-347) Establishment Name Address ID/FEI Business Operations KBI Biopharma, Inc. 034248380 analysis(80203-347) Establishment Name Address ID/FEI Business Operations Genzyme Corporation 050424395 pack(80203-347) , label(80203-347) Establishment Name Address ID/FEI Business Operations Eurofins Biopharma Product Testing Munich GmbH 313046917 analysis(80203-347) Establishment Name Address ID/FEI Business Operations Sanofi-Aventis Deutschland GmbH 313218430 pack(80203-347) , label(80203-347)
Trademark Results [Enjaymo]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ENJAYMO 88255430 not registered Live/Pending |
Bioverativ Therapeutics Inc. 2019-01-09 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.