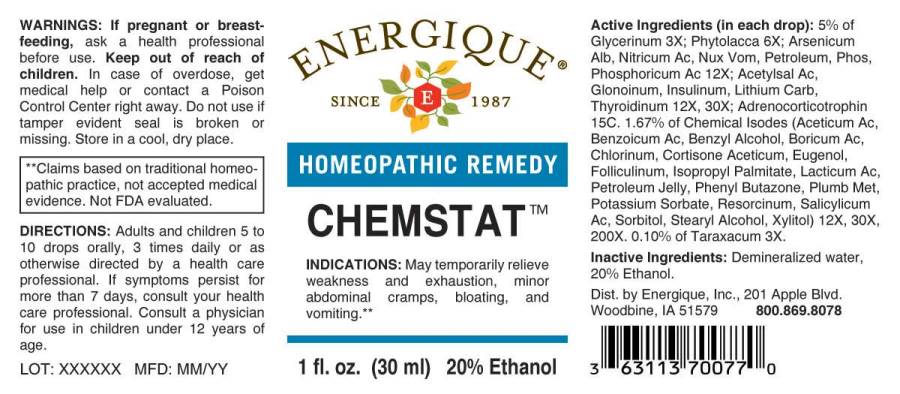
CHEMSTAT (glycerinum, taraxacum officinale, phytolacca decandra, arsenicum album, nitricum acidum, nux vomica, petroleum, phosphoricum acidum, phosphorus, acetylsalicylicum acidum, glonoinum, insulinum (human), lithium carbonicum, thyroidinum- suis, aceticum acidum, benzoicum acidum, benzyl alcohol, boricum acidum, chlorinum, cortisone aceticum, eugenol, folliculinum, isopropyl palmitate, lacticum acidum, petroleum jelly, phenyl butazone, plumbum metallicum, potassium sorbate, resorcinum, salicylicum acidum, liquid
Chemstat by
Drug Labeling and Warnings
Chemstat by is a Homeopathic medication manufactured, distributed, or labeled by Energique, Inc., Apotheca Company. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
ACTIVE INGREDIENTS:
(in each drop): 5% of Glycerinum 3X; Phytolacca Decandra 6X; Arsenicum Album 12X, Nitricum Acidum 12X, Nux Vomica 12X, Petroleum 12X, Phosphorus 12X, Phosphoricum Acidum 12X; Acetylsalicylicum Acidum 12X, 30X, Glonoinum 12X, 30X, Insulinum (Human) 12X, 30X, Lithium Carbonicum 12X, 30X, Thyroidinum (Suis) 12X, 30X; Adrenocorticotrophin 15C. 1.67% of Aceticum Acidum 12X, 30X, 200X, Benzoicum Acidum 12X, 30X, 200X, Benzyl Alcohol 12X, 30X, 200X, Boricum Acidum 12X, 30X, 200X, Chlorinum 12X, 30X, 200X, Cortisone Aceticum 12X, 30X, 200X, Eugenol 12X, 30X, 200X, Folliculinum 12X, 30X, 200X, Isopropyl Palmitate 12X, 30X, 200X, Lacticum Acidum 12X, 30X, 200X, Petroleum Jelly 12X, 30X, 200X, Phenyl Butazone 12X, 30X, 200X, Plumbum Metallicum 12X, 30X, 200X, Potassium Sorbate 12X, 30X, 200X, Resorcinum 12X, 30X, 200X, Salicylicum Acidum 12X, 30X, 200X, Sorbitol 12X, 30X, 200X, Stearyl Alcohol 12X, 30X, 200X, Xylitol 12X, 30X, 200X. 0.10% of Taraxacum Officinale 3X.
- INDICATIONS:
- WARNINGS:
- KEEP OUT OF REACH OF CHILDREN:
- DIRECTIONS:
- INDICATIONS:
- INACTIVE INGREDIENTS:
- QUESTIONS:
- PACKAGE LABEL DISPLAY:
-
INGREDIENTS AND APPEARANCE
CHEMSTAT
glycerinum, taraxacum officinale, phytolacca decandra, arsenicum album, nitricum acidum, nux vomica, petroleum, phosphoricum acidum, phosphorus, acetylsalicylicum acidum, glonoinum, insulinum (human), lithium carbonicum, thyroidinum (suis), aceticum acidum, benzoicum acidum, benzyl alcohol, boricum acidum, chlorinum, cortisone aceticum, eugenol, folliculinum, isopropyl palmitate, lacticum acidum, petroleum jelly, phenyl butazone, plumbum metallicum, potassium sorbate, resorcinum, salicylicum acidum, liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 44911-0556 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 3 [hp_X] in 1 mL TARAXACUM OFFICINALE (UNII: 39981FM375) (TARAXACUM OFFICINALE - UNII:39981FM375) TARAXACUM OFFICINALE 3 [hp_X] in 1 mL PHYTOLACCA AMERICANA ROOT (UNII: 11E6VI8VEG) (PHYTOLACCA AMERICANA ROOT - UNII:11E6VI8VEG) PHYTOLACCA AMERICANA ROOT 6 [hp_X] in 1 mL ARSENIC TRIOXIDE (UNII: S7V92P67HO) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC TRIOXIDE 12 [hp_X] in 1 mL NITRIC ACID (UNII: 411VRN1TV4) (NITRIC ACID - UNII:411VRN1TV4) NITRIC ACID 12 [hp_X] in 1 mL STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 12 [hp_X] in 1 mL KEROSENE (UNII: 1C89KKC04E) (KEROSENE - UNII:1C89KKC04E) KEROSENE 12 [hp_X] in 1 mL PHOSPHORIC ACID (UNII: E4GA8884NN) (PHOSPHORIC ACID - UNII:E4GA8884NN) PHOSPHORIC ACID 12 [hp_X] in 1 mL PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 12 [hp_X] in 1 mL ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 12 [hp_X] in 1 mL NITROGLYCERIN (UNII: G59M7S0WS3) (NITROGLYCERIN - UNII:G59M7S0WS3) NITROGLYCERIN 12 [hp_X] in 1 mL INSULIN HUMAN (UNII: 1Y17CTI5SR) (INSULIN HUMAN - UNII:1Y17CTI5SR) INSULIN HUMAN 12 [hp_X] in 1 mL LITHIUM CARBONATE (UNII: 2BMD2GNA4V) (LITHIUM CATION - UNII:8H8Z5UER66) LITHIUM CARBONATE 12 [hp_X] in 1 mL THYROID (UNII: 6RV024OAUQ) (SUS SCROFA THYROID - UNII:6RV024OAUQ) THYROID 12 [hp_X] in 1 mL ACETIC ACID (UNII: Q40Q9N063P) (ACETIC ACID - UNII:Q40Q9N063P) ACETIC ACID 12 [hp_X] in 1 mL BENZOIC ACID (UNII: 8SKN0B0MIM) (BENZOIC ACID - UNII:8SKN0B0MIM) BENZOIC ACID 12 [hp_X] in 1 mL BENZYL ALCOHOL (UNII: LKG8494WBH) (BENZYL ALCOHOL - UNII:LKG8494WBH) BENZYL ALCOHOL 12 [hp_X] in 1 mL BORIC ACID (UNII: R57ZHV85D4) (BORIC ACID - UNII:R57ZHV85D4) BORIC ACID 12 [hp_X] in 1 mL CHLORINE (UNII: 4R7X1O2820) (CHLORINE - UNII:4R7X1O2820) CHLORINE 12 [hp_X] in 1 mL CORTISONE ACETATE (UNII: 883WKN7W8X) (CORTISONE - UNII:V27W9254FZ) CORTISONE ACETATE 12 [hp_X] in 1 mL EUGENOL (UNII: 3T8H1794QW) (EUGENOL - UNII:3T8H1794QW) EUGENOL 12 [hp_X] in 1 mL ESTRONE (UNII: 2DI9HA706A) (ESTRONE - UNII:2DI9HA706A) ESTRONE 12 [hp_X] in 1 mL ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) (ISOPROPYL PALMITATE - UNII:8CRQ2TH63M) ISOPROPYL PALMITATE 12 [hp_X] in 1 mL LACTIC ACID, DL- (UNII: 3B8D35Y7S4) (LACTIC ACID, DL- - UNII:3B8D35Y7S4) LACTIC ACID, DL- 12 [hp_X] in 1 mL POTASSIUM SORBATE (UNII: 1VPU26JZZ4) (SORBIC ACID - UNII:X045WJ989B) POTASSIUM SORBATE 12 [hp_X] in 1 mL PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 12 [hp_X] in 1 mL PHENYLBUTAZONE (UNII: GN5P7K3T8S) (PHENYLBUTAZONE - UNII:GN5P7K3T8S) PHENYLBUTAZONE 12 [hp_X] in 1 mL LEAD (UNII: 2P299V784P) (LEAD - UNII:2P299V784P) LEAD 12 [hp_X] in 1 mL RESORCINOL (UNII: YUL4LO94HK) (RESORCINOL - UNII:YUL4LO94HK) RESORCINOL 12 [hp_X] in 1 mL SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 12 [hp_X] in 1 mL STEARYL ALCOHOL (UNII: 2KR89I4H1Y) (STEARYL ALCOHOL - UNII:2KR89I4H1Y) STEARYL ALCOHOL 12 [hp_X] in 1 mL SORBITOL (UNII: 506T60A25R) (SORBITOL - UNII:506T60A25R) SORBITOL 12 [hp_X] in 1 mL XYLITOL (UNII: VCQ006KQ1E) (XYLITOL - UNII:VCQ006KQ1E) XYLITOL 12 [hp_X] in 1 mL CORTICOTROPIN (UNII: K0U68Q2TXA) (CORTICOTROPIN - UNII:K0U68Q2TXA) CORTICOTROPIN 15 [hp_C] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 44911-0556-1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 10/07/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 10/07/2020 Labeler - Energique, Inc. (789886132) Registrant - Apotheca Company (844330915) Establishment Name Address ID/FEI Business Operations Apotheca Company 844330915 manufacture(44911-0556) , api manufacture(44911-0556) , label(44911-0556) , pack(44911-0556)
Trademark Results [Chemstat]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CHEMSTAT 86815500 not registered Live/Pending |
Instrumentation Laboratory Company 2015-11-10 |
 CHEMSTAT 86653468 4931059 Live/Registered |
Stern & Stern Industries, Inc. 2015-06-05 |
 CHEMSTAT 75178731 2099415 Live/Registered |
Starpoint Software Inc. 1996-10-08 |
 CHEMSTAT 73831792 1628852 Live/Registered |
CHEMAX, INC. 1989-10-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.