ALLERGY RELIEF- diphenhydramine hcl capsule, liquid filled
Allergy Relief by
Drug Labeling and Warnings
Allergy Relief by is a Otc medication manufactured, distributed, or labeled by Topco Associates, LLC, LNK International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient (in each liquid-filled capsule)
- Purpose
- Uses
-
Warnings
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- trouble urinating due to an enlarged prostate gland
- to make a child sleepy
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal display panel
TopCare
Health™NDC: 36800-658-08
COMPARE TO BENADRYL®
DYE-FREE ALLERGY LIQUI-GELS®
ACTIVE INGREDIENT*DYE-FREE
Allergy Relief
DIPHENHYDRAMINE HCl 25 mg - AntihistamineRELIEF OF:
Sneezing
Runny nose
Itchy, Watery Eyes
Itchy, Nose or Throat
Our Pharmacists Recommend
24 SOFTGELS**
** Liquid Filled Capsulesactual size
DISTRIBUTED BY TOPCO ASSOCIATES LLC
ELK GROVE VILLAGE, IL 60007
©TOPCO LNKA0219 QUESTIONS? 1-888-423-0139
topcare@topco.com www.topcarebrand.comPRODUCT OF CHINA
PACKAGED AND QUALITY ASSURED IN THE USA*This product is not manufactured or distributed by
McNeil Consumer Healthcare, owner of the registered
trademark Benadryl® Dye-Free Allergy LIQUI-GELS®.50844 ORG061465808
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS
OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR
SHOWS ANY SIGNS OF TAMPERING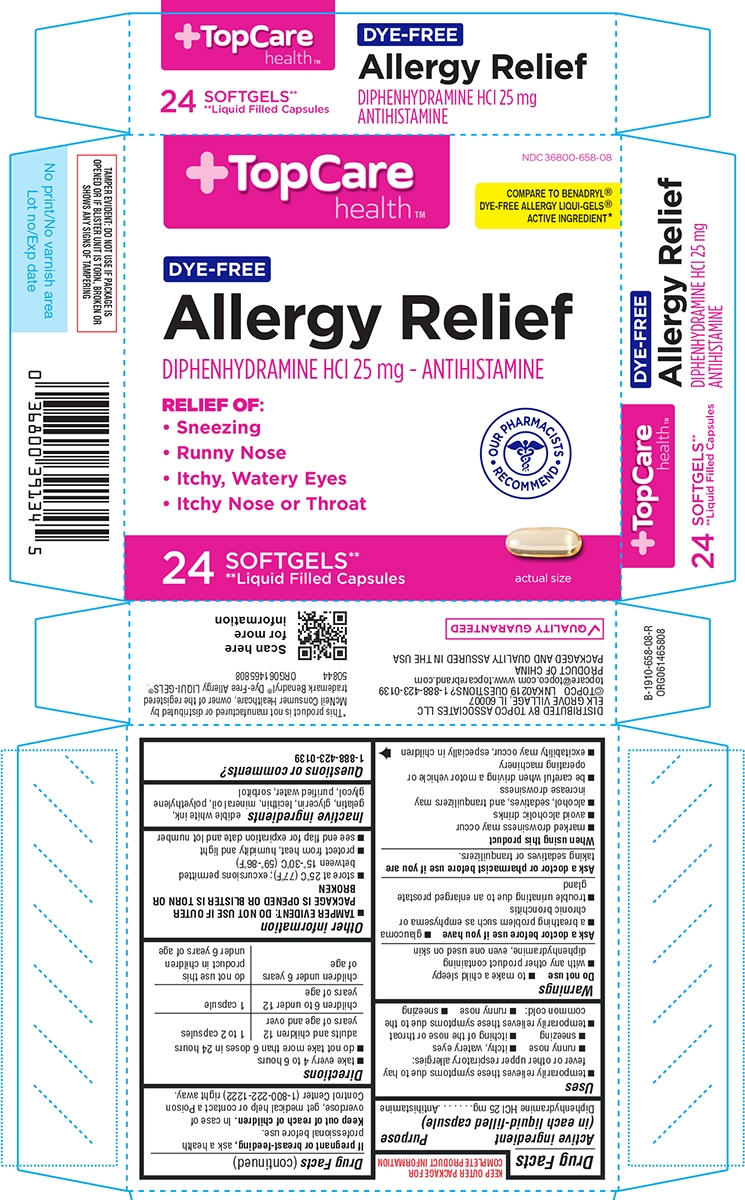
Topcare 44-658
-
INGREDIENTS AND APPEARANCE
ALLERGY RELIEF
diphenhydramine hcl capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 36800-658 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) MINERAL OIL (UNII: T5L8T28FGP) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) Product Characteristics Color YELLOW (clear) Score no score Shape OVAL Size 15mm Flavor Imprint Code 658 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 36800-658-08 2 in 1 CARTON 06/15/2019 1 12 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part341 06/15/2019 Labeler - Topco Associates, LLC (006935977) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 PACK(36800-658)
Trademark Results [Allergy Relief]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ALLERGY RELIEF 98236984 not registered Live/Pending |
Dmytro Kononenko 2023-10-24 |
 ALLERGY RELIEF 90457167 not registered Live/Pending |
American Textile Company, Inc. 2021-01-10 |
 ALLERGY RELIEF 78838437 3358249 Live/Registered |
Meshbesher Health Corporation 2006-03-16 |
 ALLERGY RELIEF 76619855 3066888 Live/Registered |
AMERICAN TEXTILE COMPANY 2004-11-09 |
 ALLERGY RELIEF 74668018 not registered Dead/Abandoned |
NaturaLife Corporation 1995-05-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.