EXXUA- gepirone tablet, extended release
EXXUA by
Drug Labeling and Warnings
EXXUA by is a Prescription medication manufactured, distributed, or labeled by Fabre Kramer Pharmaceuticals, Inc., Mission Pharmacal Company. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use EXXUA safely and effectively. See full prescribing information for EXXUA.
EXXUA (gepirone) extended-release tablets, for oral use
Initial U.S. Approval: 2023WARNING: SUICIDAL THOUGHTS AND BEHAVIORS
See full prescribing information for complete boxed warning.
Increased risk of suicidal thinking and behavior in pediatric and young adult patients taking antidepressants. Closely monitor for worsening and emergence of suicidal thoughts and behaviors ( 5.1). EXXUA is not approved for use in pediatric patients ( 8.4).
INDICATIONS AND USAGE
EXXUA is indicated for the treatment of major depressive disorder (MDD) in adults ( 1).
DOSAGE AND ADMINISTRATION
- Correct electrolyte abnormalities and perform electrocardiogram (ECG) prior to initiating treatment with EXXUA. Do not initiate EXXUA if QTc is > 450 msec ( 2.1).
- Perform ECGs during dosage titration and periodically during treatment ( 2.1).
- The recommended starting dose is 18.2 mg administered orally once daily with food at approximately the same time each day ( 2.2, 2.3).
- Depending on clinical response and tolerability, the dosage may be increased to 36.3 mg once daily on Day 4. Dosage may be further titrated to 54.5 mg once daily after Day 7 and to 72.6 mg once daily after an additional week ( 2.3).
- Geriatric patients: Recommended starting dosage is 18.2 mg once daily. Dosage may be increased to 36.3 mg after 7 days ( 2.4).
- Renal Impairment (creatinine clearance < 50 mL/min): Recommended starting dosage is 18.2 mg once daily. Dosage may be increased to 36.3 mg once daily after 7 days ( 2.5, 8.6).
- Moderate Hepatic Impairment (Child Pugh B): Dosage may be increased to 36.3 mg once daily after 7 days ( 2.6, 8.7).
- Adjust EXXUA dose by 50% when a moderate CYP3A4 inhibitor is administered (
2.7).
DOSAGE FORMS AND STRENGTHS
Extended-release tablets: 18.2 mg, 36.3 mg, 54.5 mg, and 72.6 mg ( 3).
CONTRAINDICATIONS
- Known hypersensitivity to gepirone or components of EXXUA ( 4).
- Prolonged QTc interval > 450 msec at baseline ( 4).
- Congenital long QT syndrome ( 4).
- Concomitant use of strong CYP3A4 inhibitors ( 4).
- Severe hepatic impairment ( 4).
- Use with an MAOI or within 14 days of stopping treatment with EXXUA. Do not use EXXUA within 14 days of discontinuing an MAOI ( 4).
WARNINGS AND PRECAUTIONS
- QT Interval Prolongation: EXXUA prolongs the QTc. Correct electrolyte abnormalities. Perform ECGs prior to initiation, during dose titration, and periodically during treatment with EXXUA. Monitor ECGs more frequently when EXXUA is used concomitantly with drugs known to prolong the QT interval, in patients who develop QTc ≥ 450 msec during treatment or are at significant risk of developing torsade de pointes. Do not escalate dosage if QTc > 450 msec ( 2.1, 5.2, 7).
- Serotonin Syndrome: Increased risk when co-administered with other serotonergic agents. If serotonin syndrome occurs, discontinue EXXUA and initiate supportive measures ( 5.3).
- Activation of Mania/Hypomania: Screen patients for bipolar disorder ( 5.4).
ADVERSE REACTIONS
Most common adverse reactions (incidence of ≥5% and at least twice incidence of placebo) were dizziness, nausea, insomnia, abdominal pain, and dyspepsia ( 6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Fabre-Kramer at 713-975-6900 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Strong CYP3A4 inducers: Reduces EXXUA exposure. Avoid concomitant use ( 7).
USE IN SPECIFIC POPULATIONS
Pregnancy: Third trimester use may increase the risk for persistent pulmonary hypertension and symptoms of poor adaptation (respiratory distress, temperature instability, feeding difficulty, hypotonia, irritability) in the neonate ( 8.1).
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 9/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: SUICIDAL THOUGHTS AND BEHAVIORS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Recommendations Prior to Initiating and During Treatment with EXXUA
2.2 Important Administration Instructions
2.3 Recommended Dosage
2.4 Recommended Dosage in Geriatric Patients
2.5 Recommended Dosage in Patients with Renal Impairment
2.6 Recommended Dosage in Patients with Hepatic Impairment
2.7 Dosage Modifications for Concomitant Use with CYP3A4 Inhibitors
2.8 Switching a Patient to or from a Monoamine Oxidase Inhibitor (MAOI) Antidepressant
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Suicidal Thoughts and Behaviors in Adolescents and Young Adults
5.2 QT Prolongation
5.3 Serotonin Syndrome
5.4 Activation of Mania/Hypomania
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: SUICIDAL THOUGHTS AND BEHAVIORS
Antidepressants increased the risk of suicidal thoughts and behaviors in pediatric and young adult patients in short-term studies. Closely monitor all antidepressant-treated patients for clinical worsening and emergence of suicidal thoughts and behaviors [see Warnings and Precautions (5.1)]. EXXUA is not approved for use in pediatric patients [see Use in Specific Populations (8.4)]
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Recommendations Prior to Initiating and During Treatment with EXXUA
Electrocardiogram and Electrolyte Monitoring
- Correct electrolyte abnormalities prior to initiating EXXUA. In patients with electrolyte abnormalities, or who are receiving diuretics or glucocorticoids, or who have a history of hypokalemia or hypomagnesemia, also monitor electrolytes during dose titration and periodically during treatment with EXXUA [see Warnings and Precautions (5.2)].
- Perform an electrocardiogram (ECG) prior to initiating EXXUA, during dosage titration, and periodically during treatment.
- Do not initiate EXXUA if QTc is > 450 msec at baseline.
- Monitor ECGs more frequently if EXXUA is used:
- concomitantly with drugs known to prolong the QT interval
- in patients who develop QTc ≥ 450 msec during treatment
- in patients with a significant risk of developing torsade de pointes [see Warnings and Precautions (5.2)and Drug Interactions (7)].
- Do not escalate the EXXUA dosage if the QTcF is > 450 msec [see Warnings and Precautions (5.2)].
Bipolar Disorder, Mania, and Hypomania Screening
Screen patients for a personal or family history of bipolar disorder, mania, or hypomania prior to initiating treatment with EXXUA [see Warnings and Precautions (5.3)].
2.2 Important Administration Instructions
Take EXXUA orally with food at approximately the same time each day [see Clinical Pharmacology (12.3)]. Swallow tablets whole. Do not split, crush, or chew EXXUA.
2.3 Recommended Dosage
The recommended starting dosage of EXXUA is 18.2 mg once daily. Based on clinical response and tolerability, the dosage may be increased to 36.3 mg orally once daily on Day 4 and further titrated to 54.5 mg orally once daily after Day 7 and to 72.6 mg orally once daily after an additional week. The maximum recommended daily dosage of EXXUA is 72.6 mg once daily.
2.4 Recommended Dosage in Geriatric Patients
The recommended starting dosage of EXXUA in geriatric patients is 18.2 mg orally once daily. Based on clinical response and tolerability, the dosage may be increased to maximum recommended dosage of 36.3 mg orally once daily after Day 7 [see Use in Specific Populations (8.5)and Clinical Pharmacology (12.3)].
2.5 Recommended Dosage in Patients with Renal Impairment
The recommended starting dosage of EXXUA in patients with creatinine clearance < 50 mL/min is 18.2 mg orally once daily. Based on clinical response and tolerability, the dosage may be increased to the maximum recommended dosage of 36.3 mg orally once daily after Day 7 [see Use in Specific Populations (8.6)and Clinical Pharmacology (12.3)].
The recommended dosage in patients with creatinine clearance ≥ 50 mL/min is the same as in patients with normal renal function [see Use in Specific Populations (8.6)and Clinical Pharmacology (12.3)].
2.6 Recommended Dosage in Patients with Hepatic Impairment
The recommended starting dose of EXXUA in patients with moderate (Child-Pugh B) hepatic impairment is 18.2 mg once daily. Based on clinical response and tolerability, the dosage may be increased to the maximum recommended dosage of 36.3 mg orally once daily after Day 7 [see Use in Specific Populations (8.7)and Clinical Pharmacology (12.3)].
EXXUA is contraindicated in patients with severe (Child-Pugh C) hepatic impairment [see Use in Specific Populations (8.7)and Clinical Pharmacology (12.3)].
The recommended dosage in patients with mild (Child-Pugh A) hepatic impairment is the same as patients with normal hepatic function.
2.7 Dosage Modifications for Concomitant Use with CYP3A4 Inhibitors
Reduce the EXXUA dose by 50% when used concomitantly with a moderate CYP3A4 inhibitor [see Drug Interactions (7)].
EXXUA is contraindicated in patients receiving strong CYP3A4 inhibitors [see Contraindications (4)and Drug Interactions (7)].
2.8 Switching a Patient to or from a Monoamine Oxidase Inhibitor (MAOI) Antidepressant
At least 14 days must elapse between discontinuation of an MAOI intended to treat depression and initiation of therapy with EXXUA. Conversely, at least 14 days must be allowed after stopping EXXUA before starting an MAOI antidepressant [see Contraindications (4)and Drug Interactions (7)].
-
3 DOSAGE FORMS AND STRENGTHS
EXXUA is available as extended-release tablets in the following strengths, as gepirone base:
- 18.2 mg: pink, modified rectangular, with “FK” debossed on one side and “1” on the other side.
- 36.3 mg: off-white, modified rectangular, with “FK” debossed on one side and “7” on the other side.
- 54.5 mg: yellow, modified rectangular, with “FK” debossed on one side and “11” on the other side.
- 72.6 mg: red-brown, modified rectangular, with “FK” debossed on one side and “17” on the other side.
-
4 CONTRAINDICATIONS
EXXUA is contraindicated in patients:
- with known hypersensitivity to gepirone or components of EXXUA [see Adverse Reactions (6.1)].
- with prolonged QTc interval > 450 msec at baseline [see Warnings and Precautions (5.2)].
- with congenital long QT syndrome [see Warnings and Precautions (5.2)].
- receiving concomitant strong CYP34A inhibitors [see Warnings and Precautions (5.2)and Drug Interactions (7)].
- with severe hepatic impairment [see Warnings and Precautions (5.2)and Use in Specific Populations (8.7)] .
- taking, or within 14 days of stopping, MAOIs due to the risk of serious and possibly fatal drug interactions, including hypertensive crisis and serotonin syndrome
[see
Warnings and Precautions (5.3)and
Drug Interactions (7)]
. Starting EXXUA in a patient treated with reversible MAOIs such as linezolid or intravenous methylene blue is also contraindicated.
-
5 WARNINGS AND PRECAUTIONS
5.1 Suicidal Thoughts and Behaviors in Adolescents and Young Adults
In pooled analyses of placebo-controlled trials of antidepressant drugs (SSRIs and other antidepressant classes) that included approximately 77,000 adult patients, and 4,500 pediatric patients, the incidence of suicidal thoughts and behaviors in antidepressant-treated patients aged 24 years and younger was greater than in placebo-treated patients.
There was considerable variation in risk of suicidal thoughts and behaviors among drugs, but there was an increased risk identified in young patients for most drugs studied. There were differences in absolute risk of suicidal thoughts and behaviors across the different indications, with the highest incidence in patients with MDD. The drug-placebo differences in the number of cases of suicidal thoughts and behaviors per 1000 patients treated are provided in Table 1.
Table 1 Risk Differences of the Number of Patients with Suicidal Thoughts and Behaviors in the Pooled Placebo-Controlled Trials of Antidepressants in Pediatric* and Adult Patients Age Range Drug-Placebo Difference in Number of Patients with Suicidal Thoughts or Behaviors per 1000 Patients Treated Increases Compared to Placebo < 18 years old 14 additional patients 18-24 years old 5 additional patients Decreases Compared to Placebo 25-64 years old 1 fewer patient ≥ 65 years old 6 fewer patients *EXXUA is not approved for use in pediatric patients. It is unknown whether the risk of suicidal thoughts and behaviors in children, adolescents, and young adults extends to longer-term use, i.e., beyond four months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with MDD that the use of antidepressants can delay the recurrence of depression and that depression itself is a risk factor for suicidal thoughts and behaviors.
Monitor all antidepressant-treated patients for clinical worsening and emergence of suicidal thoughts and behaviors, especially during the initial few months of drug therapy, and at times of dosage changes. Counsel family members or caregivers of patients to monitor for changes in behavior and to alert the healthcare provider. Consider changing the therapeutic regimen, including possibly discontinuing EXXUA, in patients whose depression is persistently worse, or who are experiencing emergent suicidal thoughts or behaviors.
5.2 QT Prolongation
EXXUA prolongs the QTc interval [see Clinical Pharmacology (12.2)].
EXXUA is contraindicated in patients with congenital long QT syndrome and in patients with severe hepatic impairment or in patients receiving concomitant strong CYP3A4 inhibitors as they increase EXXUA plasma concentrations [see Contraindications (4), Drug Interactions (7), and Use in Specific Populations (8.7)].
Do not initiate EXXUA if QTc is > 450 msec at baseline [see Dosage and Administration (2.1)and Contraindications (4)] .
Correct electrolyte abnormalities prior to EXXUA initiation. In patients with electrolyte abnormalities, who are receiving diuretics or glucocorticoids, or have a history or hypokalemia or hypomagnesemia, also monitor electrolytes during dose titration and periodically during treatment with EXXUA.
Perform an ECG prior to EXXUA initiation, during dosage titration, and periodically during treatment.
Monitor patients with ECGs more frequently.
- If EXXUA is used concomitantly with drugs known to prolong the QT interval [see Drug Interactions (7)].
- In patients who develop QTc ≥ 450 msec during treatment with EXXUA. Do not escalate the EXXUA dosage if QTcF is > 450 msec [see Dosage and Administration (2.1)].
- In patients with a significant risk of developing torsade de pointes, including those with uncontrolled or significant cardiac disease, recent myocardial infarction, heart failure, unstable angina, bradyarrhythmias, uncontrolled hypertension, high degree atrioventricular block, severe aortic stenosis, or uncontrolled hypothyroidism.
Reduce the EXXUA dosage when used concomitantly with moderate CYP3A4 inhibitors, as they may increase EXXUA concentrations [seeDosage and Administration (2.7)and Drug Interactions (7)] .
5.3 Serotonin Syndrome
Concomitant use of EXXUA with SSRIs or tricyclic antidepressants may cause serotonin syndrome, a potentially life-threatening condition with changes including altered mental status, hypertension, restlessness, myoclonus, hyperthermia, hyperreflexia, diaphoresis, shivering, and tremor [see Drug Interactions (7)].
The concomitant use of EXXUA with MAOIs is contraindicated. In addition, do not initiate EXXUA in a patient being treated with MAOIs such as linezolid or intravenous methylene blue. If it is necessary to initiate treatment with an MAOI such as linezolid or intravenous methylene blue in a patient taking EXXUA discontinue EXXUA before initiating treatment with the MAOI [see Contraindications (4)and Drug Interactions (7)].
If concomitant use of EXXUA with other serotonergic drugs is clinically warranted, inform patients of the increased risk for serotonin syndrome and monitor for symptoms. Discontinue EXXUA and/or concomitant serotonergic drug immediately if the above symptoms occur and initiate supportive symptomatic treatment.
5.4 Activation of Mania/Hypomania
Antidepressant treatment can precipitate a manic, mixed, or hypomanic manic episode. The risk appears to be increased in patients with bipolar disorder or who have risk factors for bipolar disorder. Prior to initiating treatment with EXXUA, screen patients for a history of bipolar disorder and the presence of risk factors for bipolar disorder (e.g., family history of bipolar disorder, suicide, or depression) [see Dosage and Administration (2.1)]. EXXUA is not approved for use in treating bipolar depression.
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Suicidal Thoughts and Behaviors in Adolescents and Young Adults [see Warnings and Precautions (5.1)]
- QT Prolongation [see Warnings and Precautions (5.2)]
- Serotonin Syndrome
[see Warnings and Precautions (5.3)]
-
Activation of Mania or Hypomania [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
During premarketing assessment, multiple doses of EXXUA were administered to 1,976 adult patients with major depressive disorder (MDD) in controlled phase 2 and 3 clinical studies, including 1,639 patients in placebo-controlled phase 2 and 3 trials in MDD, with 237 patients exposed for over six months. The population treated with EXXUA in the pooled placebo-controlled studies ranged from 15 to 78 years of age, was 34% male and 66% female, and was 80% Caucasian, 11% Black, and 9% other race.
The adverse reaction data below are based on two placebo-controlled, flexible-dose, clinical studies (Study 1, Study 2) in which either EXXUA 18.2 mg to 72.6 mg (n=226) or placebo (n=230) was administered to adult patients with MDD during an 8-week double-blind treatment period [see Clinical Studies (14)]. Study 1 had a median age of 39 years and were 61% female, 73% Caucasian, 9% Black, 2% Asian, and 16% Other (Hispanic or Native American). Study 2 had a median age of 39 years and were 69% female, 65% Caucasian, 23% Black, 1% Asian, and 11% Hispanic.
In Study 1 and Study 2, 7% (15/226) of patients treated with EXXUA and 3% (6/230) of patients receiving placebo discontinued treatment due to an adverse reaction. The most common reactions leading to discontinuation for patients taking EXXUA were dizziness and nausea.
The most common adverse reactions (≥ 5% and twice the incidence of placebo) in EXXUA-treated patients were dizziness, nausea, insomnia, abdominal pain, and dyspepsia.
Table 2presents the adverse reactions that occurred at an incidence of ≥ 2% of patients treated with EXXUA and at a higher incidence than in the placebo-treated patients.
Table 2 Adverse Reactions that Occurred in ≥ 2% of Patients Treated with EXXUA and Greater than the Incidence in Placebo-Treated Patients in Pooled MDD Studies (Study 1 and Study 2) Adverse Reaction Placebo
(N=230)
(%)EXXUA
( 18.2 mg to 76.2 mg)
(N=226)
(%)Dizziness* 10 49 Nausea 13 35 Headache* 20 31 Feeling Sleepy or Tired* 14 15 Insomnia* 5 14 Diarrhea 9 10 Upper Respiratory Tract Infection 7 8 Dry Mouth 5 8 Vomiting 4 7 Abdominal Pain* 3 7 Dyspepsia 2 6 Increased Appetite 3 5 Constipation 3 4 Nasopharyngitis 3 4 Nasal Congestion 2 4 Paresthesia 1 4 Hyperhidrosis 0 4 Palpitations 0 4 Weight Increased 1 3 Agitation 0 3 Feeling Jittery 0 3 Heart Rate Increased 0 2 Lethargy 0 2 *The following terms were combined:
Dizziness=Lightheadedness, Dizziness, Dizziness Postural.
Headache=Headache, Sinus Headache, Tension Headache.
Feeling Sleepy or Tired=Fatigue, Sedation, Somnolence.
Insomnia=Initial Insomnia, Insomnia, Middle Insomnia, Terminal Insomnia.
Abdominal Pain=Abdominal Discomfort, Abdominal Pain, Abdominal Pain Upper.Other Adverse Reactions Observed in Clinical Studies
The following is a list of adverse reactions that occurred at an incidence of < 2% in MDD patients treated with EXXUA and at least greater than placebo in Study 1 and Study 2: breast tenderness, confusional state, dyspnea, edema peripheral energy increased, feeling abnormal, hypoesthesia, poor quality sleep, and thinking abnormal.
Additional Adverse Reaction Observed in Clinical Studies
Hypersensitivity reactions including rash, pruritus, and urticaria were reported in clinical studies with EXXUA.
-
7 DRUG INTERACTIONS
Table 3displays clinically important drug interactions with EXXUA.
Table 3 Clinically Important Drug Interactions with EXXUA CYP3A4 Inhibitors Clinical Impact Strong CYP3A4 Inhibitors
Concomitant use of EXXUA with a strong CYP3A4 inhibitor increases EXXUA exposure by ~ 5-fold [see Clinical Pharmacology (12.3)].
Moderate CYP3A4 Inhibitors
Concomitant use with a moderate CYP3A4 inhibitor increases EXXUA exposure by ~ 2.6-fold [see Clinical Pharmacology (12.3)].Intervention Strong CYP3A4 Inhibitors
EXXUA is contraindicated in patients taking strong CYP3A4 inhibitors [see Dosage and Administration (2.7), Contraindications (4), and Warnings and Precautions (5.2)].
Moderate CYP3A4 Inhibitors
If EXXUA is used with a moderate CYP3A4 inhibitor, reduce the dosage of EXXUA [see Dosage and Administration (2.7)and Warnings and Precautions (5.2)].Monoamine Oxidase Inhibitors (MAOIs) Clinical Impact Concomitant use EXXUA with MAOIs increases the risk of serotonin syndrome. Intervention EXXUA is contraindicated in patients taking MAOIs, including MAOIs such as linezolid or intravenous methylene blue or in patients who have taken MAOIs within the preceding 14 days.
Allow at least 14 days after stopping EXXUA before starting an MAOI [see Dosage and Administration (2.8), Contraindications (4), and Warnings and Precautions (5.3)].Drugs that Prolong the QTc Interval
Clinical Impact Concomitant use of drugs that prolong the QTc interval may add to the QTc prolonging effects of EXXUA and increase the risk of cardiac arrhythmias. Intervention Monitor patients with ECGs more frequently if EXXUA is administered with other drugs known to prolong QT interval [see Warnings and Precautions (5.2)]. CYP3A4 Inducers Clinical Impact Concomitant use of EXXUA with a strong CYP3A4 inducers reduces EXXUA exposure by 20- to 29-fold [see Clinical Pharmacology (12.3)]. Intervention Avoid concomitant use of EXXUA in patients taking strong CYP3A4 inducers. Other Serotonergic Drugs
Clinical Impact Concomitant use of EXXUA and serotonergic drugs increases the risk of serotonin syndrome. Intervention Monitor for symptoms of serotonin syndrome when EXXUA is used concomitantly with other drugs that may affect the serotonergic neurotransmitter systems. If serotonin syndrome occurs, consider discontinuation of EXXUA and/or concomitant serotonergic drug [see Warnings and Precautions (5.3)]. -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to antidepressants, including EXXUA, during pregnancy. Healthcare providers are encouraged to register patients by calling the National Pregnancy Registry for Antidepressants at 1-866-961-2388 or visiting online at https://womensmentalhealth.org/research/pregnancyregistry/antidepressants/.
Risk Summary
Based on animal reproduction studies, gepirone has been shown to have adverse effects on embryo/fetal and postnatal development. In rats, increased mortality during the first 4 days after birth and persistent reduction in body weight through lactation and weaning were observed at all doses and increased still births were seen with a no observed adverse effect level (NOAEL) at 3 times the maximum recommended human dose (MRHD) on a mg/m 2basis. In embryofetal development studies in rats and rabbits, decreased embryofetal growth, body weights and lengths, with accompanying skeletal variations were seen with a NOAEL at 9 and 12 times the MRHD on a mg/m 2basis, respectively (see Data). There are insufficient clinical data on gepirone use during pregnancy to evaluate for a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. There are clinical considerations regarding neonates exposed to serotonergic antidepressants during the third trimester of pregnancy (see Clinical Considerations and Data). There are risks associated with untreated depression during pregnancy (see Clinical Considerations). Consider if the risks outweigh the benefits of treatment with gepirone during pregnancy.
The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Disease-associated Maternal and/or Embryofetal Risk
Women who discontinue antidepressants during pregnancy are more likely to experience a relapse of major depression than women who continue antidepressants. This finding is from a prospective, longitudinal study of 201 pregnant women with a history of major depressive disorder who were euthymic and taking antidepressants at the beginning of pregnancy. Consider the risk of untreated depression when discontinuing or changing treatment with antidepressant medication during pregnancy and postpartum.
Fetal/Neonatal Adverse Reactions
Neonates exposed to other serotonergic antidepressants in the third trimester have developed complications requiring prolonged hospitalization, respiratory support, and tube feeding. Complications can arise immediately upon delivery. Reported clinical findings have included respiratory distress, cyanosis, apnea, seizures, temperature instability, feeding difficulty, vomiting, hypoglycemia, hypotonia, hypertonia, hyperreflexia, tremors, jitteriness, irritability, and constant crying. These findings are consistent with either a direct toxic effect of SSRIs or possibly a drug discontinuation syndrome. It should be noted that, in some cases, the clinical picture is consistent with serotonin syndrome [See Warnings and Precautions (5.3)].
Data
Human Data
Exposure during late pregnancy to serotonergic antidepressants may have an increased risk for persistent pulmonary hypertension of the newborn (PPHN). PPHN occurs in 1- 2 per 1,000 live births in the general population and is associated with substantial neonatal morbidity and mortality.
Animal Data
In embryofetal development studies, oral administration of gepirone to pregnant rats (75, 150, and 300 mg/kg) or pregnant rabbits (50, 100, and 200 mg/kg) during the period of organogenesis resulted in decreased embryofetal growth, body weights and lengths, with accompanying skeletal variations at mid and high doses; the mid doses are 18 and 24 times the MRHD on a mg/m 2basis in rats and rabbits, respectively. No malformations were seen in these studies. The developmental NOAEL was 9 and 12 times the MRHD on a mg/m 2basis in rats and rabbits, respectively.
When pregnant rats were treated with gepirone (10, 20, and 40 mg/kg) from late gestation through weaning, decreased birth weights were seen at mid and high doses; the mid dose is twice the MRHD. Increased offspring mortality during the first 4 days after birth and persistent reduction in body weight were observed at all doses; the lowest dose is approximately equal to the MRHD on a mg/m 2basis. The no-effect dose for fetal effects was not determined in this study.
When gepirone was administered orally to male and female rats prior to and throughout mating, gestation, and lactation at doses of 5, 27, 64, and 150 mg/kg/day. Increased still births were seen at ≥64 mg/kg. Early postnatal mortality was increased at 150 mg/kg (18 times the MRHD on a mg/m 2basis). The NOAEL (27 mg/kg) for still births was associated with a maternal dose 3 times the MRHD on a mg/m 2basis. Fetal weights were decreased at 27 mg/kg (3 times the MRHD on a mg/m 2basis) and fetal lengths were decreased at 64 mg/kg (8 times the MRHD on a mg/m 2basis) and above. Pup weights were decreased at birth, throughout lactation and weaning, and until at least 14 weeks of age, with delays of some developmental landmarks, at 64 mg/kg and above. The NOAEL for growth and development (5 mg/kg) was associated with a maternal dose below the MRHD on a mg/m 2basis.
8.2 Lactation
Risk Summary
There are no data on the presence of gepirone in human milk, the effects on the breastfed infant, or the effects on milk production. Gepirone is present in rat milk. When a drug is present in animal milk, it is likely that the drug will be present in human milk. There are reports of breastfed infants exposed to other serotonergic antidepressants experiencing irritability, restlessness, excessive somnolence, decreased feeding, and weight loss (see Clinical Considerations). The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for EXXUA and any adverse effects on the breastfed infant from EXXUA or from the underlying maternal condition.
Clinical Considerations
Monitor breastfeeding infants for adverse reactions, such as irritability, restlessness, excessive somnolence, decreased feeding, and weight loss.
8.4 Pediatric Use
The safety and effectiveness of EXXUA in pediatric patients have not been established for the treatment of MDD.
Efficacy was not demonstrated in two 8-week, randomized, placebo-controlled trials in 426 pediatric patients 7 to 17 years of age with MDD. The primary efficacy endpoint was change from double-blind baseline to Week 8 on the Children’s Depression Rating Scale-Revised (CDRS-R) measure. The effect of treatment with EXXUA was not significantly different from placebo. In the pediatric trial patients, there was a higher occurrence of vomiting in pediatric patients (13%) compared adults (6.6%).
Antidepressants, such as EXXUA, increase the risk of suicidal thoughts and behaviors in pediatric patients [see Warnings and Precautions (5.1)].
Juvenile Animal Toxicity Data
In a juvenile animal study, male and female rats were treated with gepirone once daily with oral doses of 0, 10, 40, and 70 mg/kg, from postnatal day 14 to 42. Increased motor activity and impaired performance in the Morris water maze were observed at 40 and 70 mg/kg after a two-week recovery period. The no observed adverse effect level (NOAEL) for the neurobehavioral development effect was 10 mg/kg. When the animals were mated after a 3-week recovery period, increased pre-implantation loss was observed for mated pairs treated with 70 mg/kg. The NOAEL for this finding was 40 mg/kg/day.
8.5 Geriatric Use
Of the 1,639 patients exposed to EXXUA in placebo-controlled clinical studies of MDD, 0.7% (12 patients) were 65 years of age or older and 0.2% (3 patients) were 75 years or older.
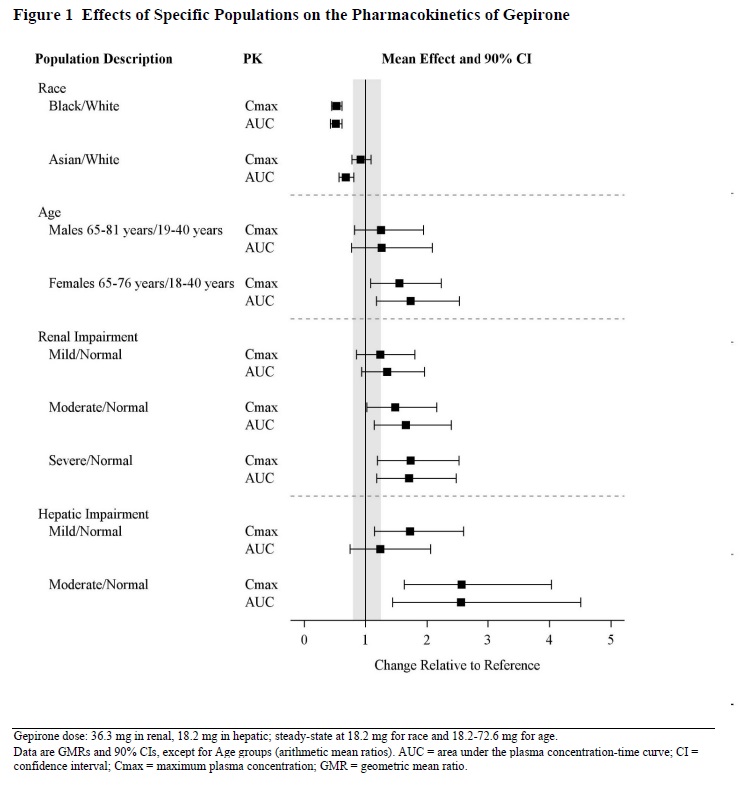
Geriatric patients (65 to 81 years of age) had higher EXXUA AUC and C maxvalues than younger adult (18 to 40 years of age) patients [see Clinical Pharmacology (12.3)]. The maximum recommended daily dosage of EXXUA in geriatric patients is lower than in younger adult patients [see Dosage and Administration (2.4)].
8.6 Renal Impairment
In patients with creatinine clearance <50 mL/min, the metabolism and excretion of EXXUA and some of its major metabolites were decreased [see Clinical Pharmacology (12.3)]. The maximum recommended daily dosage of EXXUA in patients with a creatinine clearance <50 mL/min is lower than in patients with normal renal function [see Dosage and Administration (2.5)]. The recommended dosage in patients with a creatinine clearance ≥50 mL/min is the same as in patients with normal renal function [see Dosage and Administration (2.5)].
8.7 Hepatic Impairment
In patients with mild (Child-Pugh A) hepatic impairment to moderate (Child-Pugh B) hepatic impairment, the metabolism of EXXUA and its major metabolites was decreased [see Clinical Pharmacology (12.3)]. The maximum recommended dosage of EXXUA in patients with moderate hepatic impairment is lower than in patients with normal hepatic function [see Dosage and Administration (2.6)]. The recommended dosage in patients with mild hepatic impairment is the same as in patients with normal hepatic function.
EXXUA is contraindicated in patients with severe (Child- Pugh C) hepatic impairment [see Warnings and Precautions (5.2)and Clinical Pharmacology (12.3)] .
-
10 OVERDOSAGE
In clinical studies, cases of acute ingestions up to 454 mg (6.25 times the maximum recommended dose) of EXXUA alone or in combination with other drugs, were reported. Signs and symptoms reported with overdose of EXXUA at doses up to 454 mg included vomiting and transient incomplete bundle branch block; an unknown dose of EXXUA produced altered level of consciousness and a 60-second convulsion.
No specific antidotes for EXXUA are known.
Consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdose management recommendations.
-
11 DESCRIPTION
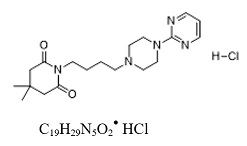
EXXUA contains gepirone, in the salt form as gepirone hydrochloride (HCl). The chemical name is 2,6- piperidinedione,4,4-dimethyl-1-[4-[4-(2-pyrimidinyl)-1-piperazinyl]butyl]-, monohydrochloride. The molecular weight of gepirone HCl is 395.93 and the structural formula is as follows:
Gepirone HCl is a white to off-white crystalline powder, which is readily soluble in water.
EXXUA is supplied as extended-release tablets for oral administration. Each extended-release tablet contains 18.2 mg, 36.3 mg, 54.5 mg, or 72.6 mg, gepirone equivalent to 20 mg, 40 mg, 60 mg, or 80 mg of gepirone HCl respectively. The extended-release tablets also contain the following inactive ingredients: colloidal silicon dioxide, Hypromellose, iron oxide (red and/or yellow colorants), magnesium stearate, and microcrystalline cellulose.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of the antidepressant effect of EXXUA is not fully understood but is thought to be related to its modulation of serotonergic activity in the CNS through selective agonist activity at 5HT1A receptors.
12.2 Pharmacodynamics
The pharmacological activity of gepirone is attributed to the parent drug and its major metabolites 3’-OH-gepirone and 1-PP. Gepirone and its 3’-OH metabolite bind to 5HT1A receptors (Ki = 38 nM and 58 nM, respectively), where they act as agonists, while the 1-PP metabolite binds to alpha2 receptors (Ki = 42 nM).
Cardiac Electrophysiology
In a thorough QT study, the largest mean increase in baseline- and placebo-corrected QTc interval with administration of 100 mg per day immediate-release formulation of gepirone was 18.4 msec (upper 90% confidence interval [CI] = 22.7 ms) on Day 1 and 16.1 msec (upper 90% CI = 20.7 ms) on Day 7. The exposure in this study was 2-fold the exposure of the maximum recommended dose [see Dosage and Administration (2.1)and Warnings and Precautions (5.2)] .
12.3 Pharmacokinetics
The pharmacokinetics of EXXUA are linear and dose proportional from 18.2 mg to 72.6 mg. Steady-state plasma concentration are typically achieved within two to four days of daily dosing.
Absorption
The absolute bioavailability is 14% to 17%. The maximal plasma EXXUA concentration (C max) after dosing is reached within 6 hours post dose (T max).
Effect of Food
After a high fat meal, T maxis reached at 3 hours. A significant effect of food has been observed on the peak plasma concentration (C max) of EXXUA and, to a lesser extent, on the total exposure (AUC0-tlast, AUC0-∞) to EXXUA. The magnitude of the food-effect was dependent of the fat content of the meal. The systemic exposure of EXXUA and major metabolites was consistently higher under fed conditions as compared to the fasted state. Gepirone Cmax after intake of low-fat (~ 200 calories) breakfast was 27% higher, after medium-fat (~500 calories) breakfast 55% higher and after a high-fat (~ 850 calories) breakfast 62% higher as compared to the fasted state. The AUC after intake of low-fat breakfast was about 14% higher, after a medium-fat breakfast 22% higher and after a high-fat breakfast 32 to 37% higher as compared to the fasted state. The effect of varying amounts of fat on C maxand AUC of the major metabolites 3-OH-gepirone and 1PP were similar to that found for gepirone [see Dosage and Administration (2.2)].
Distribution
The apparent volume of distribution of EXXUA is approximately 94.5L. The in vitroplasma protein binding in human is 72% and is not concentration dependent. The in vitroplasma protein binding for metabolite 3’-OH gepirone is 59% and 42% for 1-PP.
Elimination
The mean terminal half-life is approximately 5 hours.
Metabolism
EXXUA is extensively metabolized and both major metabolites 1-PP and 3’-OH-gepirone are present in plasma in higher concentrations than the parent compound. CYP3A4 is the primary enzyme catalyzing the metabolism of EXXUA to its major pharmacologically active metabolites.
Excretion
Following a single oral dose of [ 14C]-labeled gepirone, approximately 81% and 13% of the administered radioactivity was recovered in the urine and feces, respectively as metabolites. 60% of the gepirone was eliminated in the urine within the first 24 hours. The presence of hepatic or renal impairment did affect the apparent clearance of EXXUA.
Specific Populations
Exposures of gepirone in specific populations are summarized in Figure 1[ see Dosage and Administration (2.3, 2.4, 2.5), Contraindications (4), and Use in Specific Populations (8.5,8.6, 8.7)] .
Drug Interactions Studies
In Vivo Studies
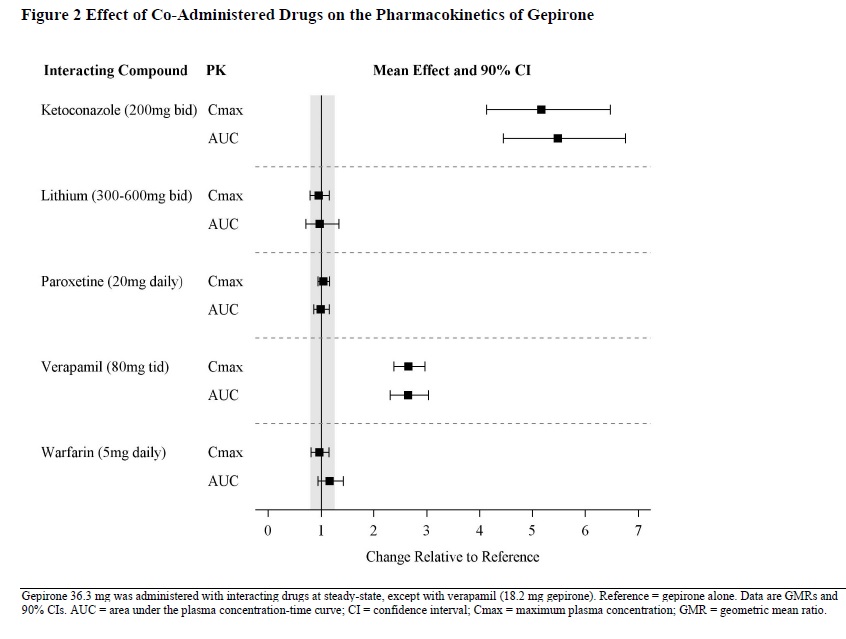
The effect of co-administered drugs on the pharmacokinetics of gepirone is summarized in Figure 2 [see Dosage and Administration (2.6)and Drug Interactions (7)].
Rifampin
The effect of multiple oral dosing of potent cytochrome P450 3A4 inducer rifampin on the steady-state pharmacokinetics of EXXUA and its major metabolites 1-PP and 3’-OH-gepirone was investigated in 24 subjects. Combined therapy with rifampin (600 mg daily) and EXXUA (20 mg for two days, then 40 mg daily) decreased C maxand AUC0-24 of EXXUA 20 times (EXXUA C maxalone 9.63 ng/mL, with rifampin 0.491 ng/mL) and 29 times (EXXUA AUC0-24 alone 123 ng·hr/mL, with rifampin 4.19 ng·hr/mL), respectively. The C maxand AUC0-24 of 3’-OH gepirone were decreased 2.5 times (3’-OH gepirone C maxalone 23.0 ng/mL, with rifampin 9.30 ng/mL) and three times (3’-OH gepirone AUC0-24 alone 371 ng·hr/mL, with rifampin 126 ng·hr/mL), respectively. There was no effect on the pharmacokinetics of 1-PP (1-PP C maxalone 6.37 ng/mL, with rifampin 6.02 ng/mL; 1-PP AUC0-24 alone 92.5 ng·hr/mL, with rifampin 81.1 ng·hr/mL).
Glyburide
Under steady-state conditions for glyburide, the addition of 36.3 mg daily of EXXUA for six days in 16 patients with stable Type II diabetes resulted in statistically significantly lower AUC (glyburide AUC 0-12alone 574.8 ng·h/mL, with EXXUA 483.0 ng·h/mL) and C max(glyburide C maxalone 121.0 ng/mL, with EXXUA 96.6 ng/mL) values for glyburide.
Drugs that Interfere with Hemostasis
Following coadministration of stable dose of warfarin (INR 1.4 to 2.0) with multiple daily doses of EXXUA, no significant effect was observed in INR, prothrombin values, or total warfarin (protein bound plus free drug) pharmacokinetics for warfarin.
Drugs that Interfere with Protein Binding
Gepirone is not highly bound to plasma protein and is not likely to be involved in interactions due to altered protein binding. In a clinical study with coadministration of EXXUA (18.2 mg) and warfarin, a highly protein-bound drug, no significant change in international normalized ratio (INR) was observed.
In Vitro Studies
Gepirone at concentrations of 0.5, 5, and 50 ng/mL was shown to have no significant impact on the plasma protein binding of chlorpromazine, desipramine, diazepam, phenytoin, prazosin, propranolol, verapamil, or warfarin. The binding of digoxin and haloperidol were decreased (at maximum) by 5% and 9%, respectively. The plasma protein binding of lidocaine appeared to be increased by 4.9% in the presence of gepirone.
Alcohol: An in vitrostudy showed dissolution rate for both 18.2 mg and 72.6 mg gepirone ER tablets decreased slightly as ethanol concentration increased in 0.01N HCl and 0.1N HCl at 0%, 5%, 10%, 20% and 40% alcohol. At 20 hours and 40% alcohol, approximately (mean) 76.8% and 80.7% were dissolved for the 18.2 mg and 72.6 mg gepirone ER tablets, respectively.
Transporter Systems:EXXUA and its metabolites are unlikely to cause clinically significant inhibition of the following transporters based on in vitro data: P-gp, BCRP, BSEP, OATP1B1, OATP1B3, OAT1, OAT3, OCT2, MATE1, and MATE2-K. As such, no clinically relevant interactions with drugs metabolized/transported by these CYP enzymes or transporters would be expected.
Enzyme systems:In addition, EXXUA has not been shown to be an inhibitor or inducer of any of the cytochrome P450 enzymes [see Clinical Pharmacology (12.3)]. Chronic administration of EXXUA is unlikely to induce the metabolism of drugs metabolized by these CYP isoforms.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
No evidence of carcinogenic potential was observed in lifetime carcinogenicity studies performed in rats and mice at doses up to 43.6 and 317.8 mg/kg/day, respectively. These doses are approximately 6 and 18 times the maximum recommended human dose (MRHD), respectively, on a mg/m 2basis.
Mutagenicity
Gepirone showed no mutagenicity in three different in vitrogenotoxicity assays (bacterial gene mutation, mammalian gene mutation, or DNA repair). No clastogenicity was observed in a rat micronucleus assay.
Impairment of Fertility
When gepirone was administered orally to male and female rats prior to and throughout mating at daily doses of 5, 27, 64, and 150 mg/kg, the latency to mating was increased at doses of 64 mg/kg (8 times the MRHD on a mg/m 2basis) and above.
-
14 CLINICAL STUDIES
The efficacy of EXXUA for the treatment for major depressive disorder (MDD) in adults was evaluated in two eight-week randomized, double-blind, placebo-controlled, flexible-dose studies in adults (age 18 to 69 years) meeting Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) criteria for MDD.
In Study 1, adult patients (18 to 69 years) who met DSM-IV diagnostic criteria for MDD were included in the trial. Patients in Study 1 had a median age of 39 years and were 61% female, 73% Caucasian, 9% Black, 2% Asian, and 16% Other (Hispanic or Native American). After an initial dosage of 18.2 mg once daily, the dosage was titrated to 36.3 mg once daily on Day 4 of treatment. The dosage could then be increased to 54.5 mg once daily after Day 7, and to 72.6 mg once daily after an additional 7 days. In patients that received EXXUA, the final daily dose of EXXUA was 72.6 mg in 64% of patients, 54.5 mg in 20% of patients, and 36.3 mg in 17% of patients.
In Study 2, adult patients (18 to 64 years) who met DSM-IV diagnostic criteria for MDD were included in the trial. Patients in Study 2 had a median age of 39 years and were 69% female, 65% Caucasian, 23% Black, 1% Asian, and 11% Hispanic. After an initial dosage of 18.2 mg daily, the dosage was titrated to 36.3 mg daily on Days 4 to 7 of treatment. The dosage could then be increased to 54.5 mg daily after Day 7, and to 72.6 mg daily after an additional 7 days. In patients that received EXXUA, the final daily dose of EXXUA was 72.6 mg in 66% of patients, 54.5 mg in 22% of patients, 36.3 mg in 10% of patients, and 18.2 mg in 2% of patients.
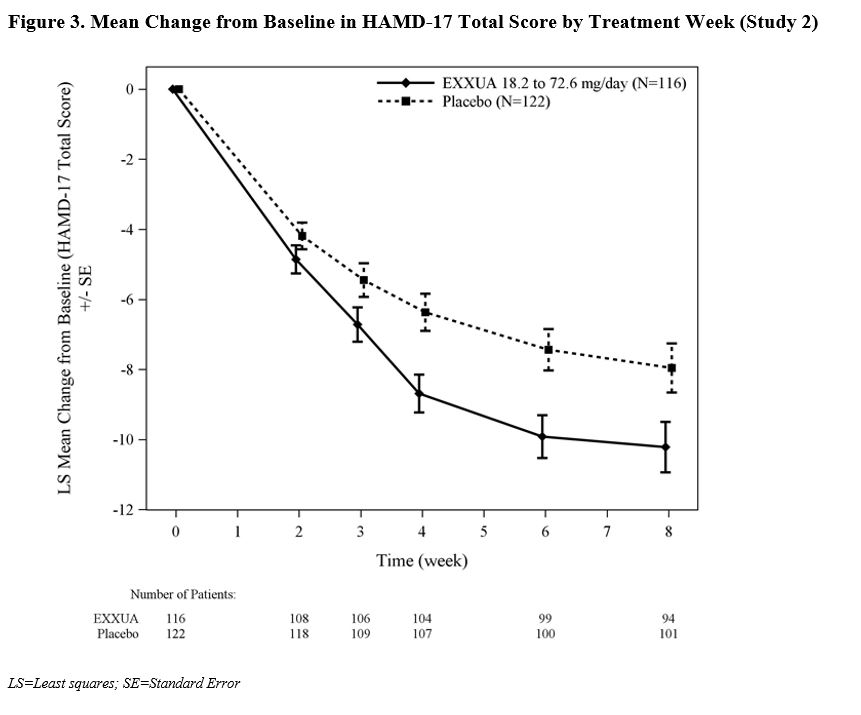
In Study 1 and Study 2, the primary efficacy measure was the change from baseline in the Hamilton Depression Rating Scale (HAMD-17) total score at Week 8. In both studies, patients in the EXXUA groups experienced statistically significantly greater improvement on the primary endpoint compared to patients in the placebo groups (see Table 4).
Table 4 Results for the Primary Endpoint: Change from Baseline in the HAMD-17 Total Score at Week 8 in Adult Patients with MDD (Study 1 and Study 2) Study
NumberTreatment Group N Mean Baseline
Score (SD)LS Mean CFB
(SE)Placebo-subtracted
Difference (95% CI)1 EXXUA
(18.2 to 72.6 mg/day)101 22.7 (2.45) -9.04 (0.78) -2.47
(-4.41, -0.53)Placebo 103 22.8 (2.51) -6.75 (0.77) 2 EXXUA
(18.2 to 72.6 mg/day)116 23.9 (2.69) -10.22 (0.75) -2.45
(-4.47, -0.43)Placebo 122 24.2 (2.93) -7.96 (0.73) N= number of patients in the primary efficacy analysis set; CFB=Change from baseline; LS=Least Squares; SD=Standard Deviation; SE=Standard Error
In Study 1, the final dose of EXXUA was 72.6, 54.5, and 36.3 mg/day in 64%, 20%, and 17% of patients, respectively.
In Study 2, the final dose of EXXUA was 72.6, 54.5, 36.3, and 18.2 mg/day in 66%, 22%, 10%, and 2% of patients, respectively.The change from baseline in HAMD-17 total score by week compared to placebo for Study 2 in shown in Figure 3.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
EXXUA (gepirone) extended-release tablets are supplied in bottles of 100 with child-resistant caps and in four dosage strengths:
-
18.2-mg Tablets– pink, modified rectangular, with “FK” debossed on one side and “1” on the other side.
Bottles of 100 NDC: 83504-150-10
-
36.3-mg Tablets– off-white, modified rectangular, with “FK” debossed on one side and “7” on the other side
Bottles of 100 NDC: 83504-151-10
-
54.5-mg Tablets– yellow, modified rectangular, with “FK” debossed on one side and “11” on the other side.
Bottles of 100 NDC: 83504-152-10
-
72.6-mg Tablets– red-brown, modified rectangular, with “FK” debossed on one side and “17” on the other side.
Bottles of 100 NDC: 83504-153-10
Storage
Store at 20°C to 25°C (68°F to 77°F); excursions are permitted between 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Protect from high humidity and moisture. -
18.2-mg Tablets– pink, modified rectangular, with “FK” debossed on one side and “1” on the other side.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Suicidal Thoughts and Behaviors
Advise patients and caregivers to look for the emergence of suicidal ideation and behavior, especially during early treatment and when the dose is adjusted up or down and instruct them to report such symptoms to their healthcare provider [see Box Warningand Warnings and Precautions (5.1)] .
QT Prolongation
Inform patients to consult their physician immediately if they feel faint, lose consciousness, or have heart palpitations [see Warnings and Precautions (5.2)]. Advise patients to inform their healthcare provider if they are taking, or plan to take, any prescription or over-the-counter medications because there is an increased risk for drug interactions with EXXUA [see Drug Interactions (7)].
Serotonin Syndrome
Caution patients about the risk of serotonin syndrome, particularly with the concomitant use of EXXUA with SSRIs or tricyclic antidepressants. Instruct patients to contact their health care provider or report to the emergency room if they experience signs or symptoms of serotonin syndrome [see Warnings and Precautions (5.3)and Drug Interactions (7)] .
Activation of Mania or Hypomania
Advise patients to observe for signs of activation of mania or hypomania and instruct them to report such symptoms to their healthcare provider [see Warnings and Precautions (5.4)].
Administration Information
Advise patients to swallow EXXUA whole and not to split, chew, or crush the tablets. Advise patients to take EXXUA at the approximately the same time every day with food [see Dosage and Administration (2.2)].
Pregnancy
Advise pregnant women to notify their healthcare provider if they become pregnant or intend to become pregnant during treatment with EXXUA.
Advise patients that there is a pregnancy registry that monitors pregnancy outcomes in women exposed to EXXUA during pregnancy .
Advise patients that EXXUA use late in pregnancy may lead to an increased risk for neonatal complications requiring prolonged hospitalization, respiratory support, tube feeding, and/or persistent pulmonary hypertension of the newborn (PPHN) [see Use in Specific Populations (8.1)].
Lactation
Advise breastfeeding individuals using EXXUA to monitor infants for excess sedation, restlessness, agitation, poor feeding and poor weight gain and to see medical care if they notice these signs [see Use in Specific Populations (8.2)].EXXUA extended-release tablets are manufactured and packaged by Mission Pharmacal Company, San Antonio, TX 78230 1355.
-
MEDICATION GUIDE
MEDICATION GUIDE
EXXUA (EKS-shoo-uh)
(gepirone)
extended-release tabletsWhat is the most important information I should know about EXXUA?
EXXUA may cause serious side effects, including:-
Increased risk of suicidal thoughts and actions.EXXUA and other antidepressant medicines may increase suicidal thoughts and actions in some people 24 years of age and younger,
especially within the first few months of treatment or when the dose is changed. EXXUA is not for use in children.
- Depression or other mental illnesses are the most important causes of suicidal thoughts or actions.
How can I watch for and try to prevent suicidal thoughts and actions in myself or family member? - Pay close attention to any changes, especially sudden changes in mood, behavior, thoughts, or feelings or if you develop suicidal thoughts or actions. This is very important when an antidepressant medicine is started or when the dose is changed.
- Call your healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings or if you develop suicidal thoughts or actions.
- Keep all follow-up visits with your healthcare provider as scheduled. Call your healthcare provider between visits as needed, especially if you have concerns about symptoms.
Call a healthcare provider or get emergency medical help right away if you or your family member has any of the following symptoms, especially if they are new, worse, or worry you: - thoughts about suicide or dying
- new or worse depression
- feeling very agitated or restless
- trouble sleeping (insomnia)
- acting aggressive, being angry, or violent
- an extreme increase in activity and talking (mania)
- attempts to commit suicide
- new or worse anxiety
- panic attacks
- new or worse irritability
- acting on dangerous impulses
- other unusual changes in behavior or mood
What is EXXUA?
EXXUA is a prescription medicine used to treat adults with a certain type of depression called major depressive disorder (MDD).
It is not known if EXXUA is safe and effective in children.- are allergic to EXXUA or any of the ingredients in EXXUA. See the end of this Medication Guide for a complete list of ingredients in EXXUA.
- have a prolonged QTc interval greater than 450 msec or congenital long QT syndrome.
- are taking medicines known as strong CYP34A inhibitors. Ask your healthcare provider if you are not sure if you are taking one of these medicines.
- have severe liver problems.
- are taking, or have stopped taking within the last 14 days, a medicine called a monoamine oxidase inhibitor (MAOI), including the antibiotic linezolid or intravenous methylene blue. Ask your healthcare provider or pharmacist if you are not sure if you take an MAOI, including the antibiotic linezolid or intravenous methylene blue.
Do not start taking an MAOI for at least 14 days after you have stopped treatment with EXXUA. Before taking EXXUA, tell your healthcare provider about all your medical conditions, including if you: - have, or have a family history of, bipolar disorder, mania, or hypomania
- have any heart problems, including heart failure, recent heart attack, high blood pressure, a slow heart rate or heart rhythm problems
- have a history of electrolyte problems, including low potassium or low magnesium
- have liver problems
- have kidney problems
- are pregnant or plan to become pregnant. EXXUA may harm your unborn baby. Taking EXXUA during the third trimester of pregnancy may cause the baby to have withdrawal symptoms, or breathing, temperature control, feeding, or other problems after birth. Talk to your healthcare provider about the risks to the baby if you take EXXUA during pregnancy.
- Tell your healthcare provider if you become pregnant or think you may be pregnant during treatment with EXXUA.
- There is a pregnancy registry for females who are exposed to EXXUA during pregnancy. The purpose of the registry is to collect information about the health of females exposed to EXXUA and their baby. If you become pregnant during treatment with EXXUA, talk to your healthcare provider about registering with the National Pregnancy Registry for Antidepressants at 1-866-961-2388 or visit online at https://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/antidepressants/.
- are breastfeeding or plan or breastfeed. It is not known if EXXUA passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby during treatment with EXXUA.
- if you breastfeed during treatment with EXXUA, call your healthcare provider if the baby develops sleepiness or fussiness, or is not feeding or gaining weight well.
Tell your healthcare provider about all the medicines you take, including prescription and medicines, vitamins, and herbal supplements.
EXXUA and other medicines may affect each other causing possible serious side effects. EXXUA may affect the way other medicines work and other medicines may affect the way EXXUA works.
Especially tell your healthcare provider if you takediuretics, corticosteroids, medicines used to treat migraine headache called triptans, or medicines used to treat mood, anxiety, psychotic or thought disorders, including selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), or tricyclic antidepressants.
Your healthcare provider can tell you if it is safe to take EXXUA with your other medicines. Do not start or stop any other medicines during treatment with EXXUA without talking to your healthcare provider first.
Know the medicines you take. Keep a list of them to show to your healthcare provider and pharmacist when you get a new medicine.How should I take EXXUA? - Take EXXUA exactly as your healthcare provider tells you to take it. Do not change the dose or stop taking EXXUA without first talking to your healthcare provider.
- Take EXXUA 1 time each day with food at about the same time each day.
- Swallow EXXUA tablets whole. Do not break, chew, crush, or dissolve tablets.
- If you miss a dose, take it as soon as you remember. If it is time for your next dose, skip the missed dose and take only your regularly scheduled dose Do not take 2 doses at the same time to make up for a missed dose.
- If you take too much EXXUA, call your healthcare provider or Poison Help Line at 1-800-222-1222 or go to the nearest hospital emergency room right away.
What are the possible side effects of EXXUA?
EXXUA may cause serious side effects, including:- See “What is the most important information I should know about EXXUA”?
- Changes in the electrical activity of your heart called QT prolongation. QT prolongation can cause irregular heartbeats that can be life-threatening or lead to death. Your healthcare provider will check the electrical activity of your heart with a test called an electrocardiogram (ECG) and will also do blood tests to check your levels of body salts (electrolytes) before and during treatment with EXXUA. Your healthcare provider may check your electrolytes more often during treatment if you have heart failure, a slow heart rate, abnormal levels of electrolytes in your blood, or if you take a medicine that can prolong the QT interval of your heartbeat. Tell your healthcare provider right away if you have an irregular heartbeat or feel dizzy, lightheaded, or faint during treatment with EXXUA.
-
Serotonin syndrome.A potentially life-threatening problem called serotonin syndrome can happen when EXXUA is taken with certain other medicines. See
“Do not take EXXUA if you.” Call your healthcare provider or go to the nearest hospital emergency room right awayif you have any of the following signs and symptoms of serotonin syndrome:
- agitation
- confusion
- fast heartbeat
- sweating
- flushing
- seizures
- nausea, vomiting, and diarrhea
- seeing or hearing things that are not real (hallucinations)
- coma
- blood pressure changes
- shaking (tremors), stiff muscles, or muscle twitching
- dizziness
- high body temperature (hyperthermia)
- loss of coordination
-
Manic episodes.Manic episodes may happen in people with bipolar disorder who take EXXUA. Symptoms may include:
- greatly increased energy
- racing thoughts
- unusually grand ideas
- talking more or faster than usual
- severe problems sleeping
- reckless behavior
- excessive happiness or irritability
The most common side effects of EXXUA include:dizziness, nausea, headache, trouble sleeping, stomach (abdominal) pain, and upset stomach.
These are not all the possible side effects of EXXUA.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store EXXUA? - Store EXXUA at room temperature between 68°F to 77°F (20°C to 25°C)
- Protect from high humidity and moisture.
-
Keep EXXUA and all medicines out of the reach of children.
General information about the safe and effective use of EXXUA
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use EXXUA for a condition for which it was not prescribed. Do not give EXXUA to other people, even if they have the same symptoms you have. It may harm them. You can ask your doctor or pharmacist for information about EXXUA that is written for health professionals.What are the ingredients in EXXUA?
Active ingredient:gepirone
Inactive ingredients:colloidal silicon dioxide, Hypromellose, iron oxide (red and/or yellow as coloring agents), magnesium stearate, and microcrystalline cellulose.
EXXUA extended-release tablets are manufactured and packaged by Mission Pharmacal Company, San Antonio, TX 78230 1355.This Medication Guide has been approved by the U.S. Food and Drug Administration. Issued 09/2023 -
Increased risk of suicidal thoughts and actions.EXXUA and other antidepressant medicines may increase suicidal thoughts and actions in some people 24 years of age and younger,
especially within the first few months of treatment or when the dose is changed. EXXUA is not for use in children.
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC83504-150-10 Rx only
EXXUA
(gepirone)
extended-release tablets
18.2 mg
Federal Law requires dispensing of EXXUA with the Medication
Guide provided with this bottle.
100 Tablets
Fabre-Kramer
Each extended-release tablet contains 18.2 mg gepirone equivalent to
20 mg gepirone hydrochloride.
Recommended Dosage: See Prescribing Information.
Dispense in a tight container with child-resistant closure.
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to
30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Protect from
high humidity and moisture.
Keep out of reach of children.
Manufactured by: Distributed by:
MISSION PHARMACAL COMPANY Fabre-Kramer Pharmaceuticals, Inc.
San Antonio, TX 78230 1355 Houston, TX 77057
L112001R0523
GTIN 000000000000000
SN 00000000000
EXP YYYY/MMM
LOT XXXXX

NDC83504-151-10 Rx only
EXXUA
(gepirone)
extended-release tablets
36.3 mg
Federal Law requires dispensing of EXXUA with the Medication
Guide provided with this bottle.
100 Tablets
Fabre-Kramer
Each extended-release tablet contains 36.3 mg gepirone equivalent to
40 mg gepirone hydrochloride.
Recommended Dosage: See Prescribing Information.
Dispense in a tight container with child-resistant closure.
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to
30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Protect from
high humidity and moisture.
Keep out of reach of children.
Manufactured by: Distributed by:
MISSION PHARMACAL COMPANY Fabre-Kramer Pharmaceuticals, Inc.
San Antonio, TX 78230 1355 Houston, TX 77057
L114001R0523
GTIN 000000000000000
SN 00000000000
EXP YYYY/MMM
LOT XXXXX
NDC83504-152-10 Rx only
EXXUA
(gepirone)
extended-release tablets
54.5 mg
Federal Law requires dispensing of EXXUA with the Medication
Guide provided with this bottle.
100 Tablets
Fabre-Kramer
Each extended-release tablet contains 54.5 mg gepirone equivalent to
60 mg gepirone hydrochloride.
Recommended Dosage: See Prescribing Information.
Dispense in a tight container with child-resistant closure.
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to
30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Protect from
high humidity and moisture.
Keep out of reach of children.
Manufactured by: Distributed by:
MISSION PHARMACAL COMPANY Fabre-Kramer Pharmaceuticals, Inc.
San Antonio, TX 78230 1355 Houston, TX 77057
L116001R0523
GTIN 000000000000000
SN 00000000000
EXP YYYY/MMM
LOT XXXXX
NDC83504-153-10 Rx only
EXXUA
(gepirone)
extended-release tablets
72.6 mg
Federal Law requires dispensing of EXXUA with the Medication
Guide provided with this bottle.
100 Tablets
Fabre-Kramer
Each extended-release tablet contains 72.6 mg gepirone equivalent to
80 mg gepirone hydrochloride.
Recommended Dosage: See Prescribing Information.
Dispense in a tight container with child-resistant closure.
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to
30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Protect from
high humidity and moisture.
Keep out of reach of children.
Manufactured by: Distributed by:
MISSION PHARMACAL COMPANY Fabre-Kramer Pharmaceuticals, Inc.
San Antonio, TX 78230 1355 Houston, TX 77057
L118001R0523
GTIN 000000000000000
SN 00000000000
EXP YYYY/MMM
LOT XXXXX
-
INGREDIENTS AND APPEARANCE
EXXUA
gepirone tablet, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 83504-151 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GEPIRONE HYDROCHLORIDE (UNII: 80C9L8EP6V) (GEPIRONE - UNII:JW5Y7B8Z18) GEPIRONE 36.3 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Product Characteristics Color white (off-white) Score no score Shape RECTANGLE Size 22mm Flavor Imprint Code FK;7 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 83504-151-10 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021164 01/01/2024 EXXUA
gepirone tablet, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 83504-150 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GEPIRONE HYDROCHLORIDE (UNII: 80C9L8EP6V) (GEPIRONE - UNII:JW5Y7B8Z18) GEPIRONE 18.2 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) FERRIC OXIDE RED (UNII: 1K09F3G675) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Product Characteristics Color pink Score no score Shape RECTANGLE Size 22mm Flavor Imprint Code FK;1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 83504-150-10 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021164 01/01/2024 EXXUA
gepirone tablet, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 83504-152 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GEPIRONE HYDROCHLORIDE (UNII: 80C9L8EP6V) (GEPIRONE - UNII:JW5Y7B8Z18) GEPIRONE 54.5 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Product Characteristics Color yellow Score no score Shape RECTANGLE Size 22mm Flavor Imprint Code FK;11 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 83504-152-10 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021164 01/01/2024 EXXUA
gepirone tablet, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 83504-153 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GEPIRONE HYDROCHLORIDE (UNII: 80C9L8EP6V) (GEPIRONE - UNII:JW5Y7B8Z18) GEPIRONE 72.6 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Product Characteristics Color red (red-brown) Score no score Shape RECTANGLE Size 22mm Flavor Imprint Code FK;17 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 83504-153-10 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021164 01/01/2024 Labeler - Fabre Kramer Pharmaceuticals, Inc. (940603442) Establishment Name Address ID/FEI Business Operations Mission Pharmacal Company 927726893 manufacture(83504-150, 83504-151, 83504-152, 83504-153)
Trademark Results [EXXUA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 EXXUA 98783944 not registered Live/Pending |
Fabre-Kramer Holdings, Inc. 2024-10-03 |
 EXXUA 90455459 not registered Live/Pending |
Fabre-Kramer Holdings, Inc. 2021-01-08 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.