ZYCLARA- imiquimod cream
Zyclara by
Drug Labeling and Warnings
Zyclara by is a Prescription medication manufactured, distributed, or labeled by Bausch Health US, LLC, Kindeva Drug Delivery Limited, Bausch Health Companies Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ZYCLARA Cream safely and effectively. See full prescribing information for ZYCLARA Cream.
ZYCLARA® (imiquimod) cream, 3.75%, for topical use
ZYCLARA® (imiquimod) cream, 2.5%, for topical use
Initial U.S. Approval: 1997INDICATIONS AND USAGE
- ZYCLARA Cream, 2.5% and 3.75% are indicated for the topical treatment of clinically typical, visible or palpable actinic keratoses (AK) of the full face or balding scalp in immunocompetent adults. (1.1)
- ZYCLARA Cream, 3.75% is also indicated for the topical treatment of external genital and perianal warts/condyloma acuminata (EGW) in patients 12 years or older. (1.2)
- Limitations of Use: Efficacy of imiquimod cream was not demonstrated for molluscum contagiosum in children 2 to 12 years of age. (1.3, 8.4)
DOSAGE AND ADMINISTRATION
- For topical use only; not for oral, ophthalmic, intra-anal, or intravaginal use. (2)
- Actinic Keratosis: Once daily to the skin of the affected area (either the entire face or balding scalp) for two 2-week treatment cycles separated by a 2-week no-treatment period. (2.1)
- External Genital Warts: Once daily to the external genital/perianal warts until total clearance or up to 8 weeks. (2.2)
DOSAGE FORMS AND STRENGTHS
- Cream: 2.5% pump; 3.75% packets or pump. (3)
CONTRAINDICATIONS
- None. (4)
WARNINGS AND PRECAUTIONS
- Intense local inflammatory reactions can occur (e.g., skin weeping, erosion). Dosing interruption may be required. (2, 5.1, 6)
- Severe local inflammatory reactions of the female external genitalia can lead to severe vulvar swelling. Severe vulvar swelling can lead to urinary retention; dosing should be interrupted or discontinued. (5.1)
- Flu-like systemic signs and symptoms including fatigue, nausea, fever, myalgias, arthralgias, and chills can occur. Dosing interruption may be required. (2.1, 2.2, 5.2, 6)
- Avoid concomitant use of ZYCLARA Cream and any other imiquimod cream because of increased risk for adverse reactions. (5.4)
ADVERSE REACTIONS
Most common adverse reactions (>4%) are local skin reactions (erythema, edema, erosion/ulceration, exudate, scabbing/crusting), headache, application site pain, application site irritation, application site pruritus, fatigue, influenza-like illness, and nausea. (6.1, 6.2)
To report SUSPECTED ADVERSE REACTIONS, contact Valeant Pharmaceuticals North America LLC at 1-800-321-4576 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 2/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Actinic Keratosis
1.2 External Genital Warts
1.3 Limitations of Use
1.4 Unevaluated Populations
2 DOSAGE AND ADMINISTRATION
2.1 Actinic Keratosis
2.2 External Genital Warts
2.3 Pump Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Local Skin Reactions
5.2 Systemic Reactions
5.3 Ultraviolet Light Exposure Risks
5.4 Increased Risk of Adverse Reactions with Concomitant Imiquimod Use
5.5 Immune Cell Activation in Autoimmune Disease
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience: Actinic Keratosis
6.2 Clinical Trials Experience: External Genital Warts
6.3 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Actinic Keratosis
14.2 External Genital Warts
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Actinic Keratosis
ZYCLARA Cream, 2.5% and 3.75% are indicated for the topical treatment of clinically typical visible or palpable, actinic keratoses (AK) of the full face or balding scalp in immunocompetent adults.
1.2 External Genital Warts
ZYCLARA Cream, 3.75% is indicated for the treatment of external genital and perianal warts (EGW)/condyloma acuminata in patients 12 years or older.
1.3 Limitations of Use
Imiquimod cream has been evaluated in children ages 2 to 12 years with molluscum contagiosum and these studies failed to demonstrate efficacy [see Use in Specific Populations (8.4)].
Treatment with ZYCLARA Cream has not been studied for prevention or transmission of human papillomavirus (HPV).
1.4 Unevaluated Populations
The safety and efficacy of ZYCLARA Cream have not been established in the treatment of:
- urethral, intravaginal, cervical, rectal, or intra-anal human papilloma viral disease.
- actinic keratosis when treated with more than one 2-cycle treatment course in the same area.
- patients with xeroderma pigmentosum.
- superficial basal cell carcinoma.
- immunosuppressed patients.
-
2 DOSAGE AND ADMINISTRATION
For topical use only; ZYCLARA Cream is not for oral, ophthalmic, intra-anal or intravaginal use.
2.1 Actinic Keratosis
ZYCLARA Cream should be applied once daily before bedtime to the skin of the affected area (either entire face or balding scalp) for two 2-week treatment cycles separated by a 2-week no-treatment period. ZYCLARA Cream should be applied as a thin film to the entire treatment area and rubbed in until the cream is no longer visible. Up to 0.5 grams (two packets or two full actuations of the pump) of ZYCLARA Cream may be applied to the treatment area at each application. ZYCLARA Cream should be left on the skin for approximately 8 hours, after which time the cream should be removed by washing the area with mild soap and water. The prescriber should demonstrate the proper application technique to maximize the benefit of ZYCLARA Cream therapy.
Patients should wash their hands before and after applying ZYCLARA Cream.
Avoid use in or on the lips and nostrils. Do not use in or near the eyes.
Local skin reactions in the treatment area are common [see Adverse Reactions (6.1)]. A rest period of several days may be taken if required by the patient's discomfort or severity of the local skin reaction. However, neither 2-week treatment cycle should be extended due to missed doses or rest periods. A transient increase in lesion counts may be observed during treatment. Response to treatment cannot be adequately assessed until resolution of local skin reactions. The patient should continue dosing as prescribed. Treatment should continue for the full treatment course even if all actinic keratoses appear to be gone. Lesions that do not respond to treatment should be carefully re-evaluated and management reconsidered.
Prescribe no more than 2 boxes (56 packets) or two 7.5 g pumps for the total 2-cycle treatment course. Partially used packets should be discarded and not reused.
2.2 External Genital Warts
Patients should apply a thin layer of ZYCLARA Cream once a day to the external genital/perianal warts until total clearance or for up to 8 weeks. Patients should use up to 0.25 grams (one packet or one full actuation of the pump) at each application, which is a sufficient amount of cream to cover the wart area. ZYCLARA Cream should be applied prior to normal sleeping hours and left on the skin for approximately 8 hours, then removed by washing the area with mild soap and water. The prescriber should demonstrate the proper application technique to maximize the benefit of ZYCLARA Cream therapy.
Patients should wash their hands before and after applying ZYCLARA Cream.
Local skin reactions at the treatment site are common [see Adverse Reactions (6.2)], and may necessitate a rest period of several days; resume treatment once the reaction subsides. Non-occlusive dressings, such as cotton gauze or cotton underwear, may be used in the management of skin reactions.
Prescribe up to 2 boxes (56 packets) or two 7.5 g pumps for the total treatment course. Use of excessive amounts of cream should be avoided. Partially used packets should be discarded and not reused.
-
3 DOSAGE FORMS AND STRENGTHS
ZYCLARA Cream, 2.5% is a white to faintly yellow cream available in pump bottles. Each pump bottle, when actuated after priming, delivers 0.235 grams of cream.
ZYCLARA Cream, 3.75% is a white to faintly yellow cream available in single-use packets and pump bottles. Each packet administers 0.25 grams of cream and each pump bottle, when actuated after priming, delivers 0.235 grams of cream (a similar amount as one packet).
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Local Skin Reactions
Intense local skin reactions including skin weeping or erosion can occur after a few applications of ZYCLARA Cream and may require an interruption of dosing [see Dosage and Administration (2) and Adverse Reactions (6)]. ZYCLARA Cream has the potential to exacerbate inflammatory conditions of the skin, including chronic graft versus host disease.
Severe local inflammatory reactions of the female external genitalia can lead to severe vulvar swelling. Severe vulvar swelling can lead to urinary retention. Dosing should be interrupted or discontinued for severe vulvar swelling.
Administration of ZYCLARA Cream is not recommended until the skin is healed from any previous drug or surgical treatment.
5.2 Systemic Reactions
Flu-like signs and symptoms may accompany, or even precede, local skin reactions and may include fatigue, nausea, fever, myalgias, arthralgias, malaise, and chills. An interruption of dosing and an assessment of the patient should be considered [see Adverse Reactions (6)].
Lymphadenopathy occurred in 2% of subjects with actinic keratosis treated with ZYCLARA Cream, 3.75% and in 3% of subjects treated with ZYCLARA Cream, 2.5% [see Adverse Reactions (6)]. This reaction resolved in all subjects by 4 weeks after completion of treatment.
5.3 Ultraviolet Light Exposure Risks
Exposure to sunlight (including sunlamps) should be avoided or minimized during use of ZYCLARA Cream. Patients should be warned to use protective clothing (e.g., a hat) when using ZYCLARA Cream. Patients with sunburn should be advised not to use ZYCLARA Cream until fully recovered. Patients who may have considerable sun exposure (e.g., due to their occupation) and those patients with inherent sensitivity to sunlight should exercise caution when using ZYCLARA Cream.
In an animal photocarcinogenicity study, imiquimod cream shortened the time to skin tumor formation [see Nonclinical Toxicology (13.1)]. The enhancement of ultraviolet carcinogenicity is not necessarily dependent on phototoxic mechanisms. Therefore, patients should minimize or avoid natural or artificial sunlight exposure.
5.4 Increased Risk of Adverse Reactions with Concomitant Imiquimod Use
Concomitant use of ZYCLARA Cream and any other imiquimod products, in the same treatment area, should be avoided since they contain the same active ingredient (imiquimod) and may increase the risk for and severity of local skin reactions.
The safety of concomitant use of ZYCLARA Cream and any other imiquimod products has not been established and should be avoided since they contain the same active ingredient (imiquimod) and may increase the risk for and severity of systemic reactions.
5.5 Immune Cell Activation in Autoimmune Disease
ZYCLARA Cream should be used with caution in patients with pre-existing autoimmune conditions because imiquimod activates immune cells [see Clinical Pharmacology (12.2)].
-
6 ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
6.1 Clinical Trials Experience: Actinic Keratosis
The data described below reflect exposure to ZYCLARA Cream or vehicle in 479 subjects enrolled in two double-blind, vehicle-controlled trials. Subjects applied up to two packets of ZYCLARA Cream or vehicle daily to the skin of the affected area (either entire face or balding scalp) for two 2-week treatment cycles separated by a 2-week no-treatment period.
Table 1: Selected Adverse Reactions Occurring in ≥2% of ZYCLARA-Treated Subjects and at a Greater Frequency than with Vehicle in the Combined Studies (AK) Adverse Reactions ZYCLARA Cream, 3.75% (N=160) ZYCLARA Cream, 2.5% (N=160) Vehicle
(N=159)Headache
10 (6%)
3 (2%)
5 (3%)
Application site pruritus
7 (4%)
6 (4%)
1 (<1%)
Fatigue
7 (4%)
2 (1%)
0
Nausea
6 (4%)
1 (1%)
2 (1%)
Influenza-like illness
1 (<1%)
6 (4%)
0
Application site irritation
5 (3%)
4 (3%)
0
Pyrexia
5 (3%)
0
0
Anorexia
4 (3%)
0
0
Dizziness
4 (3%)
1 (<1%)
0
Herpes simplex
4 (3%)
0
1 (<1%)
Application site pain
5 (3%)
2 (1%)
0
Lymphadenopathy
3 (2%)
4 (3%)
0
Oral herpes
0
4 (3%)
0
Arthralgia
2 (1%)
4 (3%)
0
Cheilitis
0
3 (2%)
0
Diarrhea
3 (2%)
2 (1%)
0
Local skin reactions were recorded as adverse reactions only if they extended beyond the treatment area, if they required any medical intervention, or they resulted in patient discontinuation from the study. The incidence and severity of selected local skin reactions are shown in Table 2.
Table 2: Local Skin Reactions in the Treatment Area in ZYCLARA-Treated Subjects as Assessed by the Investigator (AK) All Grades* (%)
Severe (%)ZYCLARA Cream, 3.75% (N=160) ZYCLARA Cream, 2.5% (N=160) Vehicle
(N=159)- * Mild, moderate, or severe
Erythema*
Severe erythema96%
25%96%
14%78%
0%Scabbing/Crusting*
Severe scabbing/crusting93%
14%84%
9%45%
0%Edema*
Severe edema75%
6%63%
4%19%
0%Erosion/Ulceration*
Severe erosion/ulceration62%
11%52%
9%9%
0%Exudate*
Severe exudate51%
6%39%
1%4%
0%Flaking/Scaling/Dryness*
Severe flaking/scaling/dryness91%
8%88%
4%77%
1%Overall, in the clinical trials, 11% (17/160) of subjects in the ZYCLARA Cream, 3.75% arm, 7% (11/160) of subjects in the ZYCLARA Cream, 2.5% arm, and 0% in the vehicle cream arm required rest periods due to adverse local skin reactions.
Other adverse reactions observed in subjects treated with ZYCLARA Cream include: application site bleeding, application site swelling, chills, dermatitis, herpes zoster, insomnia, lethargy, myalgia, pancytopenia, pruritus, squamous cell carcinoma, and vomiting.
6.2 Clinical Trials Experience: External Genital Warts
In two double-blind, placebo-controlled studies, 602 subjects applied up to one packet of ZYCLARA Cream or vehicle daily for up to 8 weeks.
The most frequently reported adverse reactions were application site reactions and local skin reactions. Selected adverse reactions are listed in Table 3.
Table 3: Selected Adverse Reactions Occurring in ≥2% of ZYCLARA-Treated Subjects and at a Greater Frequency than with Vehicle in the Combined Trials (EGW) Preferred Term ZYCLARA Cream, 3.75% (N=400) Vehicle Cream
(N=202)- * Percentage based on female population of 6/216 for ZYCLARA Cream 3.75% and 2/106 for vehicle cream
Application site pain
28 (7%)
1 (<1%)
Application site irritation
24 (6%)
2 (1%)
Application site pruritus
11 (3%)
2 (1%)
Vaginitis bacterial*
6 (3%)
2 (2%)
Headache
6 (2%)
1 (<1%)
Local skin reactions were recorded as adverse reactions only if they extended beyond the treatment area, if they required any medical intervention, or they resulted in patient discontinuation from the study. The incidence and severity of selected local skin reactions are shown in Table 4.
Table 4: Selected Local Skin Reactions in the Treatment Area Assessed by the Investigator (EGW) All Grades* (%)
Severe (%)ZYCLARA Cream, 3.75% (N=400) Vehicle Cream
(N=202)- * Mild, moderate, or severe
Erythema*
Severe erythema70%
9%27%
<1%Edema*
Severe edema41%
2%8%
0%Erosion/ulceration*
Severe erosion/ulceration36%
11%4%
<1%Exudate*
Severe exudate34%
2%2%
0%The frequency and severity of local skin reactions were similar in both genders, with the following exceptions: a) flaking/scaling occurred in 40% of men and in 26% of women and b) scabbing/crusting occurred in 34% of men and in 18% of women.
In the clinical trials, 32% (126/400) of subjects who used ZYCLARA Cream and 2% (4/202) of subjects who used vehicle cream discontinued treatment temporarily (required rest periods) due to adverse local skin reactions, and 1% (3/400) of subjects who used ZYCLARA Cream discontinued treatment permanently due to local skin/application site reactions.
Other adverse reactions reported in subjects treated with ZYCLARA Cream include: rash, back pain, application site rash, application site cellulitis, application site excoriation, application site bleeding, scrotal pain, scrotal erythema, scrotal ulcer, scrotal edema, sinusitis, nausea, pyrexia, and influenza-like symptoms.
6.3 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of imiquimod. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Application Site Disorders: tingling at the application site
Body as a Whole: angioedema
Cardiovascular: capillary leak syndrome, cardiac failure, cardiomyopathy, pulmonary edema, arrhythmias (tachycardia, supraventricular tachycardia, atrial fibrillation, palpitations), chest pain, ischemia, myocardial infarction, syncope
Endocrine: thyroiditis
Gastrointestinal System Disorders: abdominal pain
Hematological: decreases in red cell, white cell and platelet counts (including idiopathic thrombocytopenic purpura), lymphoma
Hepatic: abnormal liver function
Infections and Infestations: herpes simplex
Musculoskeletal System Disorders: arthralgia
Neuropsychiatric: agitation, cerebrovascular accident, convulsions (including febrile convulsions), depression, insomnia, multiple sclerosis aggravation, paresis, suicide
Respiratory: dyspnea
Urinary System Disorders: proteinuria, urinary retention, dysuria
Skin and Appendages: exfoliative dermatitis, erythema multiforme, hyperpigmentation, hypertrophic scar, hypopigmentation
Vascular: Henoch-Schonlein purpura syndrome
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C:
There are no adequate and well-controlled studies in pregnant women. ZYCLARA Cream should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
The animal multiples of human exposure calculations were based on daily dose comparisons for the reproductive toxicology studies described in this section and in Section 13.1. The animal multiples of human exposure were based on weekly dose comparisons for the carcinogenicity studies described in Section 13.1. For the animal multiple of human exposure ratios presented in this section and Section 13.1, the Maximum Recommended Human Dose (MRHD) was set at two packets (500 mg cream) per treatment of actinic keratosis with ZYCLARA Cream (imiquimod 3.75%, 18.75 mg imiquimod) for BSA comparison. The maximum human AUC value obtained in the treatment of external genital and perianal warts was higher than that obtained in the treatment of actinic keratosis and was used in the calculation of animal multiples of MRHD that were based on AUC comparison.
Systemic embryofetal development studies were conducted in rats and rabbits. Oral doses of 1, 5, and 20 mg/kg/day imiquimod were administered during the period of organogenesis (gestational days 6–15) to pregnant female rats. In the presence of maternal toxicity, fetal effects noted at 20 mg/kg/day (163X MRHD based on AUC comparisons) included increased resorptions, decreased fetal body weights, delays in skeletal ossification, bent limb bones, and two fetuses in one litter (2 of 1567 fetuses) demonstrated exencephaly, protruding tongues, and low-set ears. No treatment related effects on embryofetal toxicity or teratogenicity were noted at 5 mg/kg/day (28X MRHD based on AUC comparisons).
Intravenous doses of 0.5, 1, and 2 mg/kg/day imiquimod were administered during the period of organogenesis (gestational days 6–18) to pregnant female rabbits. No treatment related effects on embryofetal toxicity or teratogenicity were noted at 2 mg/kg/day (2.1X MRHD based on BSA comparisons), the highest dose evaluated in this study, or 1 mg/kg/day (115X MRHD based on AUC comparisons).
A combined fertility and peri- and postnatal development study was conducted in rats. Oral doses of 1, 1.5, 3, and 6 mg/kg/day imiquimod were administered to male rats from 70 days prior to mating through the mating period and to female rats from 14 days prior to mating through parturition and lactation. No effects on growth, fertility, reproduction, or postnatal development were noted at doses up to 6 mg/kg/day (25X MRHD based on AUC comparisons), the highest dose evaluated in this study. In the absence of maternal toxicity, bent limb bones were noted in the F1 fetuses at a dose of 6 mg/kg/day (25X MRHD based on AUC comparisons). This fetal effect was also noted in the oral rat embryofetal development study conducted with imiquimod. No treatment related effects on teratogenicity were noted at 3 mg/kg/day (12X MRHD based on AUC comparisons).
8.3 Nursing Mothers
It is not known whether imiquimod is excreted in human milk following use of ZYCLARA Cream. Because many drugs are excreted in human milk, caution should be exercised when ZYCLARA Cream is administered to nursing women.
8.4 Pediatric Use
AK is a condition not generally seen within the pediatric population. The safety and effectiveness of ZYCLARA Cream for AK in patients less than 18 years of age have not been established.
Safety and effectiveness in patients with external genital/perianal warts below the age of 12 years have not been established.
Imiquimod 5% cream was evaluated in two randomized, vehicle-controlled, double-blind trials involving 702 pediatric subjects with molluscum contagiosum (MC) (470 exposed to imiquimod; median age 5 years, range 2-12 years). Subjects applied imiquimod cream or vehicle 3 times weekly for up to 16 weeks. Complete clearance (no MC lesions) was assessed at Week 18. In Study 1, the complete clearance rate was 24% (52/217) in the imiquimod cream group compared with 26% (28/106) in the vehicle group. In Study 2, the clearance rates were 24% (60/253) in the imiquimod cream group compared with 28% (35/126) in the vehicle group. These studies failed to demonstrate efficacy.
Similar to the studies conducted in adults, the most frequently reported adverse reaction from two studies in children with molluscum contagiosum was application site reaction. Adverse events which occurred more frequently in imiquimod-treated subjects compared with vehicle-treated subjects generally resembled those seen in studies in indications approved for adults and also included otitis media (5% imiquimod vs. 3% vehicle) and conjunctivitis (3% imiquimod vs. 2% vehicle).
Erythema was the most frequently reported local skin reaction. Severe local skin reactions reported by imiquimod-treated subjects in the pediatric studies included erythema (28%), edema (8%), scabbing/crusting (5%), flaking/scaling (5%), erosion (2%) and weeping/exudate (2%).
Systemic absorption of imiquimod across the affected skin of 22 subjects aged 2 to 12 years with extensive MC involving at least 10% of the total body surface area was observed after single and multiple doses at a dosing frequency of three applications per week for 4 weeks. The investigator determined the dose applied, either 1, 2 or 3 packets per dose, based on the size of the treatment area and the subject's weight. The overall median peak serum drug concentrations at the end of Week 4 was between 0.26 and 1.06 ng/mL except in a 2-year-old female who was administered 2 packets of study drug per dose, had a Cmax of 9.66 ng/mL after multiple dosing. Children aged 2–5 years received doses of 12.5 mg (one packet) or 25 mg (two packets) of imiquimod and had median multiple-dose peak serum drug levels of approximately 0.2 or 0.5 ng/mL, respectively. Children aged 6–12 years received doses of 12.5 mg, 25 mg, or 37.5 mg (three packets) and had median multiple dose serum drug levels of approximately 0.1, 0.15, or 0.3 ng/mL, respectively. Among the 20 subjects with evaluable laboratory assessments, the median WBC count decreased by 1.4*109/L and the median absolute neutrophil count decreased by 1.42*109/L.
8.5 Geriatric Use
Of the 320 subjects treated with ZYCLARA Cream in the AK clinical studies, 150 subjects (47%) were 65 years or older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects.
Clinical studies of ZYCLARA Cream for EGW did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Of the 400 subjects treated with ZYCLARA Cream, 3.75% in the EGW clinical studies, 5 subjects (1%) were 65 years or older.
-
10 OVERDOSAGE
Topical overdosing of ZYCLARA Cream could result in an increased incidence of severe local skin reactions and may increase the risk for systemic reactions.
Hypotension was reported in a clinical trial following multiple oral imiquimod doses of >200 mg (equivalent to ingestion of the imiquimod content of more than 21 packets or pump actuations of ZYCLARA Cream, 3.75% or more than 32 pump actuations of ZYCLARA Cream, 2.5%). The hypotension resolved following oral or intravenous fluid administration.
-
11 DESCRIPTION
ZYCLARA (imiquimod) Cream, 2.5% or 3.75% is intended for topical administration. Each gram contains 25 mg or 37.5 mg of imiquimod, respectively, in a white to faintly yellow oil-in-water cream base consisting of benzyl alcohol, cetyl alcohol, glycerin, isostearic acid, methylparaben, polysorbate 60, propylparaben, purified water, sorbitan monostearate, stearyl alcohol, white petrolatum, and xanthan gum.
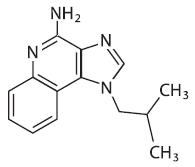
Chemically, imiquimod is 1-(2-methylpropyl)-1H-imidazol[4,5-c]quinolin-4-amine. Imiquimod has a molecular formula of C14H16N4 and a molecular weight of 240.3. Its structural formula is:
ZYCLARA (imiquimod) Cream, 3.75% comes as a premeasured packet containing 9.4 mg of imiquimod in 0.25 g of cream. ZYCLARA (imiquimod) Cream, 2.5% and 3.75% also come in pumps which dispense 5.9 mg or 8.8 mg of imiquimod, respectively, in 0.235 g of cream per full actuation of the pump after priming.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of ZYCLARA Cream in treating AK and EGW lesions is unknown.
12.2 Pharmacodynamics
The pharmacodynamics of ZYCLARA Cream are unknown.
Imiquimod is a Toll-like receptor 7 agonist that activates immune cells. Topical application to skin is associated with increases in markers for cytokines and immune cells.
12.3 Pharmacokinetics
Following dosing with two packets of ZYCLARA Cream, 3.75% once daily (18.75 mg imiquimod/day) for up to 3 weeks, systemic absorption of imiquimod was observed in all subjects when ZYCLARA Cream was applied to the face and/or scalp in 17 subjects with at least 10 AK lesions. The mean peak serum imiquimod concentration at the end of the trial was approximately 0.323 ng/mL. The median time to maximal concentrations (Tmax) occurred at 9 hours after dosing. Based on the plasma half-life of imiquimod observed at the end of the study, 29.3±17.0 hours, steady-state concentrations can be anticipated to occur by Day 7 with once-daily dosing.
Systemic absorption of imiquimod (up to 9.4 mg [one packet]) across the affected skin of 18 subjects with EGW was observed with once daily dosing for 3 weeks in all subjects. The subjects had either a minimum of 8 warts (range 8–93) or a surface area involvement of greater than 100 mm2 (range 15–620 mm2) at study entry. The mean peak serum imiquimod concentration at Day 21 was 0.488 +/- 0.368 ng/mL. The median time to maximal concentrations (Tmax) occurred 12 hours after dosing. Based on the plasma half-life of imiquimod observed at the end of the study, 24.1 +/- 12.4 hours, steady-state concentrations can be anticipated to occur by day 7 with once daily dosing. Because of the small number of subjects present (13 males, 5 females) it was not possible to select out or do an analysis of absorption based on gender/site of application.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In an oral (gavage) rat carcinogenicity study, imiquimod was administered to Wistar rats on a 2X/week (up to 6 mg/kg/day) or daily (3 mg/kg/day) dosing schedule for 24 months. No treatment-related tumors were noted in the oral rat carcinogenicity study up to the highest doses tested in this study of 6 mg/kg administered 2X/week in female rats (7.1X MRHD based on weekly AUC comparisons), 4 mg/kg administered 2X/week in male rats (6.1X MRHD based on weekly AUC comparisons) or 3 mg/kg administered 7X/week to male and female rats (12X MRHD based on weekly AUC comparisons).
In a dermal mouse carcinogenicity study, imiquimod cream (up to 5 mg/kg/application imiquimod or 0.3% imiquimod cream) was applied to the backs of mice 3X/week for 24 months. A statistically significant increase in the incidence of liver adenomas and carcinomas was noted in high-dose male mice compared to control male mice (21X MRHD based on weekly AUC comparisons). An increased number of skin papillomas was observed in vehicle cream control group animals at the treated site only.
In a 52-week dermal photocarcinogenicity study, the median time to onset of skin tumor formation was decreased in hairless mice following chronic topical dosing (3X/week; 40 weeks of treatment followed by 12 weeks of observation) with concurrent exposure to UV radiation (5 days per week) with vehicle alone. No additional effect on tumor development beyond the vehicle effect was noted with the addition of the active ingredient, imiquimod, to the vehicle cream.
Imiquimod revealed no evidence of mutagenic or clastogenic potential based on the results of five in vitro genotoxicity tests (Ames assay, mouse lymphoma L5178Y assay, Chinese hamster ovary cell chromosome aberration assay, human lymphocyte chromosome aberration assay and SHE cell transformation assay) and three in vivo genotoxicity tests (rat and hamster bone marrow cytogenetics assay and a mouse dominant lethal test).
Daily oral administration of imiquimod to rats, throughout mating, gestation, parturition and lactation, demonstrated no effects on growth, fertility or reproduction, at doses up to 25X MRHD based on AUC comparisons.
-
14 CLINICAL STUDIES
14.1 Actinic Keratosis
In two double-blind, randomized, vehicle-controlled clinical studies, 479 subjects with AK were treated with ZYCLARA Cream, 3.75%, ZYCLARA Cream, 2.5%, or vehicle cream. Studies enrolled subjects 18 years of age or older with 5 to 20 typical visible or palpable AK lesions of the face or scalp. Study cream was applied to either the entire face (excluding ears) or balding scalp once daily for two 2-week treatment cycles separated by a 2-week no-treatment period. Subjects then continued in the study for an 8-week follow-up period during which they returned for clinical observations and safety monitoring. Study subjects ranged from 36 to 90 years of age and 54% had Fitzpatrick skin type I or II. All ZYCLARA Cream-treated subjects were Caucasians.
On a scheduled dosing day, up to two packets of the study cream were applied to the entire treatment area prior to normal sleeping hours and left on for approximately 8 hours. Efficacy was assessed by AK lesion counts at the 8-week post-treatment visit. All AKs in the treatment area were counted, including baseline lesions as well as lesions which appeared during therapy.
Complete clearance required absence of any lesions including those that appeared during therapy in the treatment area. Complete and partial clearance rates are shown in the tables below. Partial clearance rate was defined as the percentage of subjects in whom the number of baseline AKs was reduced by 75% or more. The partial clearance rate was measured relative to the numbers of AK lesions at baseline.
Table 5: Rate of Subjects with Complete Clearance at 8 Weeks Post-Treatment ZYCLARA Cream, 3.75% ZYCLARA Cream, 2.5% Vehicle
CreamStudy AK1
26% (21/81)
23% (19/81)
3% (2/80)
Study AK2
46% (36/79)
38% (30/79)
10% (8/79)
Table 6: Rate of Subjects with Partial Clearance (≥75%) at 8 Weeks Post-Treatment ZYCLARA Cream, 3.75% ZYCLARA Cream, 2.5% Vehicle
CreamStudy AK1
46% (37/81)
42% (34/81)
19% (15/80)
Study AK2
73% (58/79)
54% (43/79)
27% (21/79)
During the course of treatment, 86% (138/160) of ZYCLARA Cream, 3.75% subjects and 84% (135/160) of ZYCLARA Cream, 2.5% subjects experienced a transient increase in lesions evaluated as actinic keratoses relative to the number present at baseline within the treatment area.
14.2 External Genital Warts
In two double-blind, randomized, placebo-controlled clinical studies, 601 subjects with EGW were treated with 3.75% imiquimod cream, or a matching placebo cream. Studies enrolled subjects aged from 15 to 81 years. The baseline wart area ranged from 6 to 5579 mm2 (median 60 mm2) and the baseline wart count ranged from 2 to 48 warts. Most subjects had two or more treated anatomic areas at baseline. Anatomic areas included: inguinal, perineal, and perianal areas (both genders); the glans penis, penis shaft, scrotum, and foreskin (in men); and the vulva (in women). Up to one packet of study cream was applied once daily. The study cream was applied to all warts prior to normal sleeping hours and left on for approximately 8 hours. Subjects continued applying the study cream for up to 8 weeks, stopping if they achieved complete clearance of all (baseline and new) warts in all anatomic areas. Subjects who achieved complete clearance of all warts at any time up to the Week 16 visit entered a 12-week follow-up period to assess recurrence.
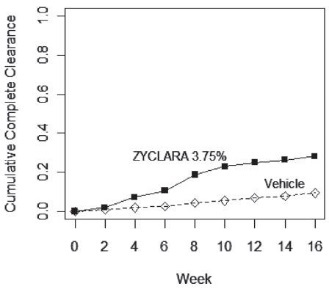
Complete clearance was defined as clearance of all warts (baseline and new) in all anatomic areas within 16 weeks from baseline. The complete clearance rates are shown in Table 7. The proportions of subjects who achieved complete clearance at or before a given week (cumulative proportion) for the combined studies are shown in Figure 1. Complete clearance rates by gender for the combined studies are shown in Table 8.
Table 7: Percent of Subjects with Complete Clearance of External Genital Warts Within 16 Weeks from Baseline ZYCLARA Cream, 3.75% Vehicle Cream Study EGW1
53/195 (27%)
10/97 (10%)
Study EGW2
60/204 (29%)
9/105 (9%)
Figure 1: Cumulative Proportion of Subjects Achieving Complete Clearance of External Genital Warts by a Given Week (Combined Studies)
Table 8: Percent of Subjects with Complete Clearance of External Genital Warts within 16 Weeks from Baseline by Gender (Combined Studies) ZYCLARA Cream, 3.75% Vehicle Cream Females
79/216 (37%)
15/106 (14%)
Males
34/183 (19%)
4/96 (4%)
Of the 113 ZYCLARA Cream, 3.75%-treated subjects who achieved complete clearance in the two studies, 17 (15%) subjects had a recurrence within 12 weeks.
No studies were conducted directly comparing the 3.75% and 5% concentrations of imiquimod cream in the treatment of external genital warts.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
ZYCLARA (imiquimod) Cream, 3.75% is white to faintly yellow in color and supplied in single-use plastic laminate packets which contain 0.25 g of the cream available as:
- Box of 28 packets containing 3.75% cream, NDC: 99207-270-28.
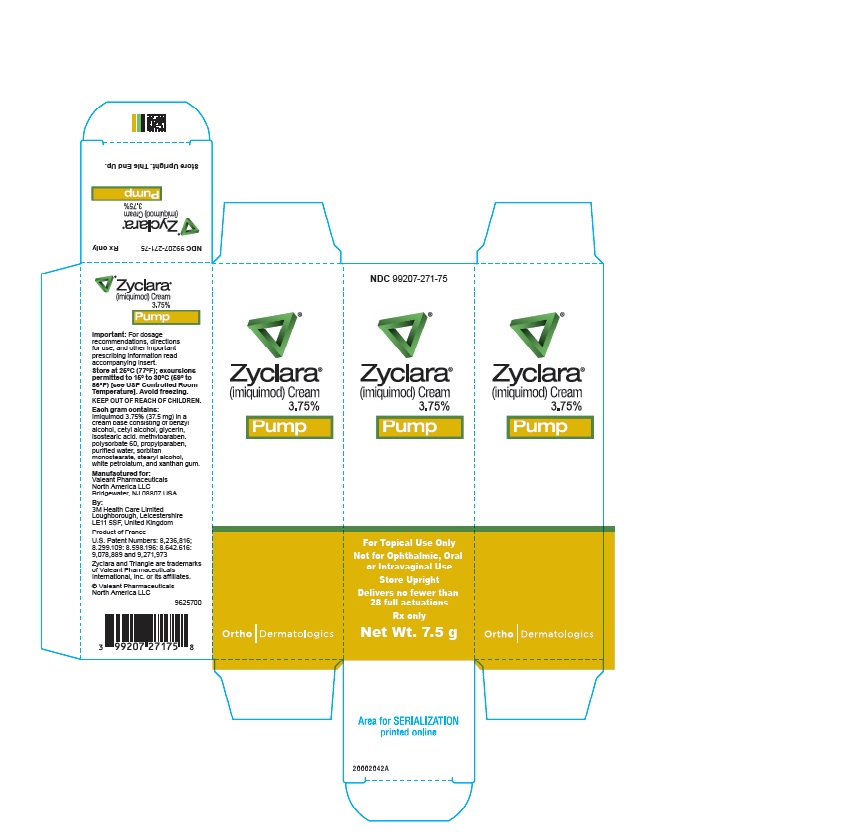
ZYCLARA (imiquimod) Cream, 2.5% and 3.75% is also supplied as white plastic 30 mL pump bottles, equipped with a white cap. The 7.5 g pump delivers no fewer than 28 full actuations.
- 7.5 g of the 2.5% cream, NDC: 99207-276-75.
- 7.5 g of the 3.75% cream, NDC: 99207-271-75.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Instructions for Administration
ZYCLARA Cream should be used as directed by a physician. ZYCLARA Cream is for external use only. Contact with the eyes, lips, nostrils, anus and vagina should be avoided [see Indications and Usage (1) and Dosage and Administration (2)].
The treatment area should not be bandaged or otherwise occluded. Partially used packets should be discarded and not reused. Pumps should be discarded after completion of a full treatment course. The prescriber should demonstrate the proper application technique to maximize the benefit of ZYCLARA Cream therapy.
It is recommended that patients wash their hands before and after applying ZYCLARA Cream.
Local Skin Reactions
Patients may experience local skin reactions during treatment with ZYCLARA Cream. Potential local skin reactions include erythema, edema, erosions/ulcerations, weeping/exudate, flaking/scaling/dryness, and scabbing/crusting. These reactions can range from mild to severe in intensity and may extend beyond the application site onto the surrounding skin. Patients may also experience application site reactions such as itching, irritation or pain [see Adverse Reactions (6)].
Local skin reactions may be of such an intensity that patients may require rest periods from treatment. Treatment with ZYCLARA Cream can be resumed after the skin reaction has subsided, as determined by the physician. However, for actinic keratosis, each treatment cycle should not be extended beyond 2 weeks due to missed doses or rest periods. For external genital warts, treatment should not be extended beyond 8 weeks due to missed doses or rest periods. Patients should contact their physician promptly if they experience any sign or symptom at the application site that restricts or prohibits their daily activity or makes continued application of the cream difficult.
Because of local skin reactions, during treatment and until healed, the treatment area is likely to appear noticeably different from normal skin. Localized hypopigmentation and hyperpigmentation have been reported following use of imiquimod cream. These skin color changes may be permanent in some patients.
Systemic Reactions
Patients may experience flu-like systemic signs and symptoms during treatment with ZYCLARA Cream. Systemic signs and symptoms may include fatigue, nausea, fever, myalgia, malaise, arthralgia, and chills [see Adverse Reactions (6)]. An interruption of dosing and an assessment of the patient should be considered.
Patients Being Treated for Actinic Keratosis (AK)
Dosing is once daily before bedtime to the skin of the affected area (entire face or balding scalp) for two 2-week treatment cycles separated by a 2-week no-treatment period. However, the treatment period should not be extended beyond two 2-week treatment cycles due to missed doses or rest periods. Treatment should continue for the full treatment course even if all actinic keratoses appear to be gone [see Dosage and Administration (2.1)].
It is recommended that patients wash their hands before and after applying ZYCLARA Cream. Before applying the cream, the patient should wash the treatment area with mild soap and water and allow the area to dry thoroughly.
It is recommended that the treatment area be washed with mild soap and water 8 hours following ZYCLARA Cream application.
Most patients using ZYCLARA Cream for the treatment of AK experience erythema, flaking/scaling/dryness, and scabbing/crusting at the application site with normal dosing [see Adverse Reactions (6.1)].
Use of sunscreen is encouraged, and patients should minimize or avoid exposure to natural or artificial sunlight (tanning beds or UVA/B treatment) while using ZYCLARA Cream [see Warnings and Precautions (5.3)].
Additional lesions may become apparent in the treatment area during treatment [see Clinical Studies (14.1)].
Patients Being Treated for External Genital Warts (EGW)
Dosing is once daily before bedtime to the skin of the affected wart areas. ZYCLARA Cream treatment should continue until there is total clearance of the genital/perianal warts or for up to 8 weeks.
It is recommended that the treatment area be washed with mild soap and water approximately 8 hours following ZYCLARA Cream application.
It is common for patients to experience local skin reactions i.e., (erythema, erosion, exudate, flaking/scaling, scabbing/crusting and edema) at the site of application or surrounding areas.
Sexual (genital, anal, oral) contact should be avoided while ZYCLARA Cream is on the skin. Application of ZYCLARA Cream in the vagina is considered internal and should be avoided. Female patients should take special care if applying the cream at the opening of the vagina because local skin reactions on the delicate moist surfaces can result in pain or swelling, and may cause difficulty in passing urine.
Uncircumcised males treating warts under the foreskin should retract the foreskin and clean the area daily.
New warts may develop during therapy, as ZYCLARA Cream is not a cure.
The effect of ZYCLARA Cream on the transmission of genital/perianal warts is unknown.
ZYCLARA Cream may weaken condoms and vaginal diaphragms, therefore concurrent use is not recommended.
Should severe local skin reactions occur, the cream should be removed by washing the treatment area with mild soap and water.
Manufactured for:
Valeant Pharmaceuticals North America LLC
Bridgewater, NJ 08807 USABy:
3M Health Care Limited
Loughborough, Leicestershire, LE11 5SF, United KingdomU.S. Patent Numbers: 8,236,816; 8,299,109; 8,598,196 8,642,616; 9,078,888 and 9,271,973 for Zyclara 3.75%
U.S. Patent Numbers: 8,222,270 and 9,370,509 for Zyclara 2.5%Zyclara is a trademark of Valeant Pharmaceuticals International, Inc. or its affiliates.
© Valeant Pharmaceuticals North America LLC -
Patient Information
ZYCLARA [zi-clar-a]
(imiquimod) Cream
Important: For use on the skin only (topical). Do not use ZYCLARA Cream in or on your eyes, mouth, anus or vagina or inside your nose.
Read the Patient Information that comes with ZYCLARA Cream before you start using it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your healthcare provider about your medical condition or treatment. If you do not understand the information, or have any questions about ZYCLARA Cream, talk with your healthcare provider or pharmacist.
What is ZYCLARA Cream?
ZYCLARA Cream, 2.5% and 3.75% are prescription medicines for skin use only (topical) to treat actinic keratosis on the face or balding scalp in adults with a normal immune system.
ZYCLARA Cream, 3.75% is a prescription medicine for use on the skin only (topical) to treat external genital and perianal warts in people 12 years and older.
It is not known if ZYCLARA Cream is safe and effective in the treatment of:
- human papilloma virus (HPV) disease of the urethra, the inside of the vagina (intravaginal), cervix, rectum, or inside of the anus (intra-anal)
- actinic keratosis, when treated with more than one 2-cycle treatment course in the same affected area
- people who have a weakened immune system
- xeroderma pigmentosum
- superficial basal cell carcinoma
It is not known if ZYCLARA Cream is safe and effective for the treatment of actinic keratosis in children less than 18 years of age.
It is not known if ZYCLARA Cream is safe and effective in children less than 12 years of age for external genital and perianal warts.
What should I tell my healthcare provider before using ZYCLARA Cream?
Before you use ZYCLARA Cream, tell your healthcare provider if you:
- have problems with your immune system.
- are being treated or have been treated for actinic keratosis with other medicines or surgery. You should not use ZYCLARA Cream until you have healed from other treatments.
- have other skin problems or sunburn.
- have any other medical conditions.
- are pregnant or planning to become pregnant. It is not known if ZYCLARA Cream can harm your unborn baby. Talk to your healthcare provider if you are pregnant or plan to become pregnant.
- are breast-feeding or plan to breast-feed. It is not known if ZYCLARA Cream passes into your breast milk and if it can harm your baby. Talk to your healthcare provider about the best way to feed your baby if you use ZYCLARA Cream.
Tell your healthcare provider about all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements.
Especially tell your healthcare provider if you have had other treatments for actinic keratosis or genital or perianal warts. ZYCLARA Cream should not be used until your skin has healed from other treatments.
How should I use ZYCLARA Cream?
Use ZYCLARA Cream exactly as your healthcare provider tells you to use it. ZYCLARA Cream is for skin use only.
- Your healthcare provider will tell you where to apply ZYCLARA Cream and how often and for how long to apply it for your condition. Do not apply ZYCLARA Cream to other areas.
- Do not use more ZYCLARA Cream than you need to cover the treatment area. Using too much ZYCLARA Cream, or using it too often, or for too long can increase your chances for having a severe skin reaction or other side effects.
- ZYCLARA Cream should be applied once a day just before your bedtime.
- Talk to your healthcare provider if you think ZYCLARA Cream is not working for you.
Applying ZYCLARA Cream:
- Wash the area where the cream will be applied with mild soap and water.
- Allow the area to dry.
- Wash your hands.
- Place the amount of cream to be used in your palm.
- Apply a thin layer of ZYCLARA Cream only to the affected area or areas to be treated. Do not use more ZYCLARA Cream than is needed to cover the treatment area.
- Rub the cream in all the way to the affected area or areas.
- After you apply ZYCLARA Cream, wash your hands well.
- Leave the cream on the affected area or areas for the time prescribed by your healthcare provider. Do not bathe or get the treated area wet before the prescribed time has passed.
- Do not leave ZYCLARA Cream on your skin longer than prescribed.
- After about 8 hours, wash the treated area or areas with mild soap and water.
- If you forget to apply ZYCLARA Cream, just apply the next dose of ZYCLARA Cream at your regular time.
How do I use ZYCLARA Cream packets?
- Open a packet of ZYCLARA Cream just before use.
- Apply ZYCLARA Cream as described above.
- After applying ZYCLARA Cream safely throw away the opened packet so that children and pets cannot get it.
- The opened packet should be thrown away even if all the ZYCLARA Cream was not completely used.
How do I use ZYCLARA Cream pump?
- Remove the cap.
- Before using the pump for the first time only, prime the pump by pressing the top of the pump all the way down repeatedly until the cream appears. The cream obtained from priming should be dispensed into a tissue and then discarded. The pump is now primed and is ready to use. You will not have to repeat this priming process during your treatment.
- When dispensing the cream, slightly tilt the pump as shown:
- Firmly press the top of the pump all the way down to dispense the cream onto your hand or fingertip.
- Apply ZYCLARA Cream as described above.
When using ZYCLARA Cream for actinic keratosis:
- Do not get ZYCLARA Cream in or near your eyes, in or on your lips, or in your nose.
- If you get ZYCLARA Cream in your mouth or in your eyes, rinse well with water right away.
- For actinic keratosis, ZYCLARA Cream should be applied once daily to the skin of the affected area (either entire face or balding scalp) for 2 weeks, then stop using for 2 weeks, then applied once daily again for 2 weeks.
- If you have been prescribed ZYCLARA Cream packets, do not use more than two packets for each daily application.
- If you have been prescribed ZYCLARA Cream pump, do not use more than two actuations of the pump for each daily application.
When using ZYCLARA Cream for external genital warts:
- Do not get ZYCLARA Cream in or on your anus or vagina.
- Apply a thin layer of ZYCLARA Cream only to the affected area or areas to be treated. Do not use more ZYCLARA Cream than is needed to cover the treatment area.
- Rub the cream into your skin until you cannot see the ZYCLARA Cream.
- ZYCLARA Cream is usually left on the skin for approximately 8 hours. Treatment should continue until the warts are completely gone or for up to 8 weeks.
- Uncircumcised males treating warts under their penis foreskin must pull their foreskin back and clean before treatment and clean daily during treatment.
- Female patients treating genital warts must be careful when applying ZYCLARA Cream around the vaginal opening. Do not put ZYCLARA Cream in your vagina.
- If you have been prescribed ZYCLARA Cream packets, do not use more than one packet for each daily application.
- If you have been prescribed ZYCLARA Cream pump, do not use more than one actuation of the pump for each daily application.
What should I avoid while using ZYCLARA Cream?
- Do not cover the treated area with bandages or other closed dressings.
- Cotton gauze dressings can be used. Cotton underwear can be worn after applying ZYCLARA Cream to the genital or perianal area.
- Do not use sunlamps or tanning beds, and avoid sunlight as much as possible during treatment with ZYCLARA Cream. Use sunscreen and wear protective clothing if you go outside during daylight.
- Do not have sexual contact including genital, anal, or oral sex when ZYCLARA Cream is on your genital or perianal skin. ZYCLARA Cream may weaken condoms and vaginal diaphragms. This means they may not work as well to prevent pregnancy.
What are the possible side effects of ZYCLARA Cream?
ZYCLARA Cream may cause serious side effects, including:
- Local Skin Reactions: Skin drainage (weeping) or breakdown of the outer layer of your skin (erosion). Swelling outside of the vagina (vulvar swelling) may happen in female patients. You should take special care if applying the cream at the opening of the vagina because local skin reactions on the delicate moist surfaces can cause pain or swelling, and may cause problems passing urine. Tell your healthcare provider if this happens.
- Flu-like symptoms: Tell your healthcare provider if you have tiredness, nausea, vomiting, fever, chills, muscle pain, and joint pain.
The most common side effects of ZYCLARA Cream include:
- local skin reactions including skin redness, scabbing, crusting, flaking, scaling, dryness, swelling
- headache
- itching at the treatment area
- tiredness
- nausea
- skin irritation
- pain at the treatment area
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of ZYCLARA Cream. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to Valeant Pharmaceuticals North America LLC at 1-800-321-4576.
How do I store ZYCLARA Cream?
- Store ZYCLARA Cream at room temperature 59° to 86°F (15° to 30°C).
- Store ZYCLARA Cream pumps upright.
- Do not freeze.
- Safely throw away ZYCLARA Cream that is out of date, unused or partially used.
Keep ZYCLARA Cream and all medicines out of the reach of children.
General Information about ZYCLARA Cream:
Medicines are sometimes prescribed for purposes other than those listed in the patient information. Do not use ZYCLARA Cream for a condition for which it was not prescribed. Do not give ZYCLARA Cream to other people, even if they have the same symptoms you have. It may harm them.
This patient information leaflet summarizes the most important information about ZYCLARA Cream. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about ZYCLARA Cream that is written for health professionals.
What are the ingredients in ZYCLARA Cream?
Active Ingredient: imiquimod
Inactive Ingredients: benzyl alcohol, cetyl alcohol, glycerin, isostearic acid, methylparaben, polysorbate 60, propylparaben, purified water, sorbitan monostearate, stearyl alcohol, white petrolatum, and xanthan gum.
This patient information leaflet has been approved by the U.S. Food and Drug Administration.Manufactured for:
Valeant Pharmaceuticals North America LLC
Bridgewater, NJ 08807 USABy:
3M Health Care Limited
Loughborough, Leicestershire, LE11 5SF, United KingdomU.S. Patent Numbers: 8,236,816; 8,299,109; 8,598,196; 8,642,616; 9,078,888 and 9,271,973 for Zyclara 3.75%
U.S. Patent Numbers: 8,222,270 and 9,370,509 for Zyclara 2.5%Zyclara is a trademark of Valeant Pharmaceuticals International, Inc. or its affiliates.
© Valeant Pharmaceuticals North America LLCRev. 02/2018
9626101
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ZYCLARA
imiquimod creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 99207-270 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength imiquimod (UNII: P1QW714R7M) (imiquimod - UNII:P1QW714R7M) imiquimod 37.5 mg in 1 g Inactive Ingredients Ingredient Name Strength ISOSTEARIC ACID (UNII: X33R8U0062) CETYL ALCOHOL (UNII: 936JST6JCN) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) PETROLATUM (UNII: 4T6H12BN9U) POLYSORBATE 60 (UNII: CAL22UVI4M) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) GLYCERIN (UNII: PDC6A3C0OX) XANTHAN GUM (UNII: TTV12P4NEE) WATER (UNII: 059QF0KO0R) BENZYL ALCOHOL (UNII: LKG8494WBH) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 99207-270-01 4 in 1 CARTON 11/28/2011 1 0.25 g in 1 PACKET; Type 0: Not a Combination Product 2 NDC: 99207-270-28 28 in 1 CARTON 11/28/2011 2 0.25 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022483 11/28/2011 ZYCLARA
imiquimod creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 99207-271 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength imiquimod (UNII: P1QW714R7M) (imiquimod - UNII:P1QW714R7M) imiquimod 37.5 mg in 1 g Inactive Ingredients Ingredient Name Strength ISOSTEARIC ACID (UNII: X33R8U0062) CETYL ALCOHOL (UNII: 936JST6JCN) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) PETROLATUM (UNII: 4T6H12BN9U) POLYSORBATE 60 (UNII: CAL22UVI4M) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) GLYCERIN (UNII: PDC6A3C0OX) XANTHAN GUM (UNII: TTV12P4NEE) WATER (UNII: 059QF0KO0R) BENZYL ALCOHOL (UNII: LKG8494WBH) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 99207-271-75 1 in 1 CARTON 01/01/2012 1 7.5 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 2 NDC: 99207-271-01 1 in 1 CARTON 01/01/2012 2 7.5 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022483 01/01/2012 ZYCLARA
imiquimod creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 99207-276 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength imiquimod (UNII: P1QW714R7M) (imiquimod - UNII:P1QW714R7M) imiquimod 2.5 mg in 1 g Inactive Ingredients Ingredient Name Strength ISOSTEARIC ACID (UNII: X33R8U0062) CETYL ALCOHOL (UNII: 936JST6JCN) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) PETROLATUM (UNII: 4T6H12BN9U) POLYSORBATE 60 (UNII: CAL22UVI4M) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) GLYCERIN (UNII: PDC6A3C0OX) XANTHAN GUM (UNII: TTV12P4NEE) WATER (UNII: 059QF0KO0R) BENZYL ALCOHOL (UNII: LKG8494WBH) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 99207-276-75 1 in 1 CARTON 02/29/2012 1 7.5 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 2 NDC: 99207-276-01 1 in 1 CARTON 02/29/2012 2 7.5 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022483 02/29/2012 Labeler - Bausch Health US, LLC (831922468) Registrant - Valeant Pharmaceuticals North America LLC (042230623) Establishment Name Address ID/FEI Business Operations 3M Health Care LTD 218829455 MANUFACTURE(99207-270, 99207-271, 99207-276) , PACK(99207-270, 99207-271, 99207-276) Establishment Name Address ID/FEI Business Operations Bausch Health Companies, Inc. 245141858 PACK(99207-271, 99207-276)
Trademark Results [Zyclara]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ZYCLARA 85721208 4280040 Live/Registered |
Medicis Pharmaceutical Corporation 2012-09-05 |
 ZYCLARA 77781257 3819643 Live/Registered |
MEDICIS PHARMACEUTICAL CORPORATION 2009-07-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.