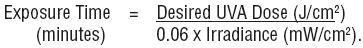METHOXSALEN capsule, liquid filled
Methoxsalen by
Drug Labeling and Warnings
Methoxsalen by is a Prescription medication manufactured, distributed, or labeled by Oceanside Pharmaceuticals, HERITAGE PHARMA LABS, INC, Carton Service Incorporated, Aphena Pharma Solutions. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
BOXED WARNING
(What is this?)
Methoxsalen with ultraviolet (UV) radiation should be used only by physicians who have special competence in the diagnosis and treatment of psoriasis and who have special training and experience in photochemotherapy. The use of psoralen and ultraviolet radiation therapy should be under constant supervision of such a physician. For the treatment of patients with psoriasis, photochemotherapy should be restricted to patients with severe, recalcitrant, disabling psoriasis which is not adequately responsive to other forms of therapy, and only when the diagnosis is certain. Because of the possibilities of ocular damage, aging of the skin, and skin cancer (including melanoma), the patient should be fully informed by the physician of the risks inherent in this therapy.
-
BOXED WARNING
(What is this?)
CAUTION: Methoxsalen Capsules, USP should not be used interchangeably with regular Oxsoralen® or 8-MOP® (Methoxsalen Hard Gelatin Capsules). This new dosage form of methoxsalen exhibits significantly greater bioavailability and earlier photosensitization onset time than previous methoxsalen dosage forms. Patients should be treated in accordance with the dosimetry specifically recommended for this product. The minimum phototoxic dose (MPD) and phototoxic peak time after drug administration prior to onset of photochemotherapy with this dosage form should be determined.
-
I. DESCRIPTION
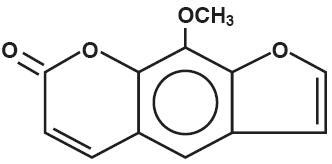
Methoxsalen Capsules, USP contains 10 mg methoxsalen (8-methoxsalen). Methoxsalen occurs as white to pale yellow crystals and can be obtained naturally from seeds of Ammi majus and roots of Heracleum Candicans or through synthesis. Methoxsalen is practically insoluble in water, freely soluble in chloroform, soluble in boiling alcohol, in acetone, in acetic acid, in propylene glycol, and in benzene, sparingly soluble in boiling water and in ether. The chemical name of methoxsalen is 9-methoxy-7H-furo [3,2-g] [1] benzopyran-7-one; its empirical formula is C12H8O4 and the molecular weight is 216.19. The structural formula is:
Methoxsalen Capsules, USP are available as soft gelatin capsules containing the following inactive ingredients: D&C yellow 10, FD&C blue 1, FD&C yellow 6, gelatin, glycerin, methylparaben,
polyethylene glycol 400, propylparaben, purified water, sorbitol solution, and titanium dioxide.
-
II. CLINICAL PHARMACOLOGY
The combination treatment regimen of psoralen (P) and ultraviolet radiation of 320-400 nm wavelength commonly referred to as UVA is known by the acronym PUVA. Skin reactivity to UVA (320-400 nm) radiation is markedly enhanced by the ingestion of methoxsalen. In a well-controlled bioavailability study, Methoxsalen Capsules, USP reached peak drug levels in the blood of test subjects between 0.5 and 4 hours (Mean = 1.8 hours) as compared to between 1.5 and 6 hours (Mean = 3.0 hours) for regular Oxsoralen when administered with 8 ounces of milk. Peak drug levels were 2- to 3- fold greater when the overall extent of drug absorption was approximately 2- fold greater for Methoxsalen Capsules, USP as compared to regular Oxsoralen Capsules. Detectable methoxsalen levels were observed up to 12 hours post dose. The drug half-life is approximately 2 hours. Photosensitivity studies demonstrate a shorter time of peak photosensitivity of 1.5 to 2.1 hours vs. 3.9 to 4.25 hours for regular Oxsoralen capsules. In addition, the mean minimal erythema dose (MED), J/cm2, for the Methoxsalen Capsules, USP is substantially less than that required for regular Oxsoralen Capsules (Levins et al., 1984 and private communication1).
Methoxsalen is reversibly bound to serum albumin and is also preferentially taken up by epidermal cells (Artuc et al., 19792). At a dose which is 6 times larger than that used in humans, it induces mixed function oxidases in the liver of mice (Mandula et al., 19783). In both mice and man, methoxsalen is rapidly metabolized. Approximately 95% of the drug is excreted as a series of metabolites in the urine within 24 hours (Pathak et al., 19774). The exact mechanism of action of methoxsalen with the epidermal melanocytes and keratinocytes is not known. The best known biochemical reaction of methoxsalen is with DNA. Methoxsalen, upon photoactivation, conjugates and forms covalent bonds with DNA which leads to the formation of both monofunctional (addition to a single strand of DNA) and bifunctional (crosslinking of psoralen to both strands of DNA) adducts (Dall’ Acqua et al., 19715; Cole, 19706; Musajo et al., 19747; Dall’ Acqua et al., 19798). Reactions with proteins have also been described (Yoshikawa, et al., 19799).
Methoxsalen acts as a photosensitizer. Administration of the drug and subsequent exposure to UVA can lead to cell injury. Orally administered methoxsalen reaches the skin via the blood and UVA penetrates well into the skin. If sufficient cell injury occurs in the skin, an inflammatory reaction occurs. The most obvious manifestation of this reaction is delayed erythema, which may not begin for several hours and peaks at 48-72 hours. The inflammation is followed, over several days to weeks, by repair which is manifested by increased melanization of the epidermis and thickening of the stratum corneum. The mechanisms of therapy are not known. In the treatment of psoriasis, the mechanism is most often assumed to be DNA photodamage and resulting decrease in cell proliferation but other vascular, leukocyte, or cell regulatory mechanisms may also be playing some role. Psoriasis is a hyperproliferative disorder and other agents known to be therapeutic for psoriasis are known to inhibit DNA synthesis.
-
III. INDICATIONS AND USAGE
Photochemotherapy (methoxsalen with long-wave UVA radiation) is indicated for the symptomatic control of severe, recalcitrant, disabling psoriasis not adequately responsive to other forms of therapy and when the diagnosis has been supported by biopsy. Methoxsalen is intended to be administered only in conjunction with a schedule of controlled doses of long-wave ultraviolet radiation.
-
IV. CONTRAINDICATIONS
A. Patients exhibiting idiosyncratic reactions to psoralen compounds.
B. Patients possessing a specific history of light-sensitive disease states should not initiate methoxsalen therapy except under special circumstances. Diseases associated with photosensitivity include lupus erythematosus, porphyria cutanea tarda, erythropoietic protoporphyria, variegate porphyria, xeroderma pigmentosum, and albinism.
C. Patients with melanoma or with a history of melanoma.
D. Patients with invasive squamous cell carcinomas.
E. Patients with aphakia because of the significantly increased risk of retinal damage due to the absence of lenses.
-
V. WARNINGS - GENERAL
A. SKIN BURNING: Serious burns from either UVA or sunlight (even through window glass) can result if the recommended dosage of the drug and/or exposure schedules are exceeded.
B. CARCINOGENICITY:
- 1. ANIMAL STUDIES: Topical or intraperitoneal methoxsalen has been reported to be a potent photocarcinogen in albino mice and hairless mice (Hakim et al., 196010). However, methoxsalen given by the oral route to Swiss albino mice suggests this agent exerts a protective effect against ultraviolet carcinogenesis; mice given 8-methoxypsoralen in their diet showed 38% ear tumors 180 days after the start of ultraviolet therapy compared to 62% for controls (O’Neal et al., 195711).
- 2. HUMAN STUDIES: A 5.7 year prospective study of 1380 psoriasis patients treated with oral methoxsalen and ultraviolet A photochemotherapy (PUVA) demonstrated that the risk of cutaneous squamous-cell carcinoma developing at least 22 months following the first PUVA exposure was approximately 12.8 times higher in the high-dose patients than in the low-dose patients (Stern et al., 197912, Stern et al., 198013, and Stern et al., 198414). The substantial dose-dependent increase was observed in patients with neither a prior history of skin cancer nor significant exposure to cutaneous carcinogens. Reduction in PUVA dosage significantly reduces the risk. No substantial dose-related increase was noted for basal cell carcinoma according to Stern et al., 198414. Increases appear greatest in patients who have pre-PUVA exposure to 1) prolonged tar and UVB treatment, 2) ionizing radiation, or 3) arsenic. Roenigk et al., 198015, studied 690 patients for up to 4 years and found no increase in the risk of non-melanoma skin cancer, although patients in this cohort had significantly less exposure to PUVA than in the Stern et al. study. Recent analysis of new data in the Stern et al. cohort (Stern et al., 199716) has shown that these patients had an elevated relative risk of contracting melanoma. The relative risk for melanoma in these patients was 2.3 (95% confidence interval 1.1 to 4.1). The risk is particularly higher in those patients who have received more than 250 PUVA treatments and in those whose treatment has spanned greater than 15 years earlier. Some patients developing melanoma did so even after having ceased PUVA therapy over 5 years earlier. These observations indicate the need for monitoring of PUVA patients for skin tumors throughout their lives. In a study in Indian patients treated for 4 years for vitiligo, 12% developed keratoses, but not cancer, in the depigmented, vitiliginous areas (Mosher, 198017). Clinically, the keratoses were keratotic papules, actinic keratosis-like macules, nonscaling dome-shaped papules, and lichenoid porokeratotic-like papules.
C. CATARACTOGENICITY:
- 1. ANIMAL STUDIES: Exposure to large doses of UVA causes cataracts in animals, and this effect is enhanced by the administration of methoxsalen (Cloud et al., 196018; Cloud et al., 196119; Freeman et al., 196920).
- 2. HUMAN STUDIES: It has been found that the concentration of methoxsalen in the lens is proportional to the serum level. If the lens is exposed to UVA during the time methoxsalen is present in the lens, photochemical action may lead to irreversible binding of methoxsalen to proteins and the DNA components of the lens (Lerman et al., 198021). However, if the lens is shielded from UVA, the methoxsalen will diffuse out of the lens in a 24-hour period (Lerman et al., 198021). Patients should be told emphatically to wear UVA-absorbing, wrap-around sunglasses for the 24-hour period following ingestion of methoxsalen whether exposed to direct or indirect sunlight in the open or through a window glass. Among patients using proper eye protection, there is no evidence for a significantly increased risk of cataracts in association with PUVA therapy (Stern et al., 197912). Thirty-five of 1380 patients have developed cataracts in the 5 years since their first PUVA treatment. This incidence is comparable to that expected in a population of this size and age distribution. No relationship between PUVA dose and cataract risk in this group has been noted.
D. ACTINIC DEGENERATION: Exposure to sunlight and/or ultraviolet radiation may result in “premature aging” of the skin.
E. BASAL CELL CARCINOMAS: Patients exhibiting multiple basal cell carcinomas or having a history of basal cell carcinomas should be diligently observed and treated.
F. RADIATION THERAPY: Patients having a history of previous x-ray therapy or grenz ray therapy should be diligently observed for signs of carcinoma.
G. ARSENIC THERAPY: Patients having a history of previous arsenic therapy should be diligently observed for signs of carcinoma.
H. HEPATIC DISEASES: Patients with hepatic insufficiency should be treated with caution since hepatic biotransformation is necessary for drug urinary excretion.
I. CARDIAC DISEASES: Patients with cardiac diseases or others who may be unable to tolerate prolonged standing or exposure to heat stress should not be treated in a vertical UVA chamber.
J. GERIATRIC PATIENTS: Caution should be used in elderly patients, especially those with a pre-existing history of cataracts, cardiovascular conditions, kidney and/or liver dysfunction, or skin cancer.
K. TOTAL DOSAGE: The total cumulative dose of UVA that can be given over long periods of time with safety has not as yet been established.
L. CONCOMITANT THERAPY: Special care should be exercised in treating patients who are receiving concomitant therapy (either topically or systemically) with known photosensitizing agents such as anthralin, coal tar or coal tar derivatives, griseofulvin, phenothiazines, nalidixic acid, fluoroquinolone antibiotics, halogenated salicylanilides (bacteriostatic soaps), sulfonamides, tetracyclines, thiazides, and certain organic staining dyes such as methylene blue, toluidine blue, rose bengal, and methyl orange.
-
VI. PRECAUTIONS
A. GENERAL - APPLICABLE TO PSORIASIS TREATMENT:
-
1. BEFORE METHOXSALEN INGESTION
Patients must not sunbathe during the 24 hours prior to methoxsalen ingestion and UV exposure. The presence of a sunburn may prevent an accurate evaluation of the patient’s response to photochemotherapy. - 2. AFTER METHOXSALEN INGESTION
- a. UVA-absorbing wrap-around sunglasses should be worn during daylight for 24 hours after methoxsalen ingestion. The protective eyewear must be designed to prevent entry of stray radiation to the eyes, including that which may enter from the sides of the eyewear. The protective eyewear is used to prevent the irreversible binding of methoxsalen to the proteins and DNA components of the lens. Cataracts form when enough of the binding occurs. Visual discrimination should be permitted by the eyewear for patient well-being and comfort.
- b. Patients must avoid sun exposure, even through window glass or cloud cover, for at least 8 hours after methoxsalen ingestion. If sun exposure cannot be avoided, the patient should wear protective devices such as a hat and gloves, and/or apply sunscreens which contain ingredients that filter out UVA radiation (e.g., sunscreens containing benzophenone and/or PABA esters which exhibit a sun protective factor equal to or greater than 15). These chemical sunscreens should be applied to all areas that might be exposed to the sun (including lips). Sunscreens should not be applied to areas affected by psoriasis until after the patient has been treated in the UVA chamber.
- 3. DURING PUVA THERAPY
- a. Total UVA-absorbing/blocking goggles mechanically designed to give maximal ocular protection must be worn. Failure to do so may increase the risk of cataract formation. A reliable radiometer can be used to verify elimination of UVA transmission through the goggles.
- b. Abdominal skin, breasts, genitalia, and other sensitive areas should be protected for approximately one-third of the initial exposure time until tanning occurs.
- c. Unless affected by disease, male genitalia should be shielded.
- 4. AFTER COMBINED METHOXSALEN/UVA THERAPY
- a. UVA-absorbing wrap-around sunglasses should be worn during daylight for 24 hours after combined methoxsalen/UVA therapy.
- b. Patients should not sunbathe for 48 hours after therapy. Erythema and/or burning due to photochemotherapy and sunburn due to sun exposure are additive.
C. LABORATORY TESTS:
- 1. Patients should have an ophthalmologic examination prior to start of therapy, and thence yearly.
- 2. Patients should have routine laboratory tests prior to the start of therapy and at regular periods thereafter if patients are on extended treatments.
F. PREGNANCY:
Animal reproduction studies have not been conducted with methoxsalen. It is also not known whether methoxsalen can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Methoxsalen should be given to a woman with reproductive capacity only if clearly needed.
G. NURSING MOTHERS:
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, either methoxsalen ingestion or nursing should be discontinued.
H. PEDIATRIC USE:
Safety in children has not been established. Potential hazards of long-term therapy include the possibilities of carcinogenicity and cataractogenicity as described in the WARNINGS-GENERAL –section as well as the probability of actinic degeneration which is also described in the WARNINGS – GENERAL section.
I. GERIATRIC USE:
Clinical studies with Methoxsalen Capsules, USP did not include sufficient numbers of subjects aged 65 and over to determine whether elderly subjects responded differently from younger subjects. Other reported clinical experience has not identified differences in response between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
1. BEFORE METHOXSALEN INGESTION
-
VII. ADVERSE REACTIONS
A. METHOXSALEN:
The most commonly reported side effect of methoxsalen alone is nausea, which occurs with approximately 10% of all patients. This effect may be minimized or avoided by instructing the patient to take methoxsalen in milk or food, or to divide the dose into two portions, taken approximately one-half hour apart. Other effects include nervousness, insomnia, and depression.
B. COMBINED METHOXSALEN/UVA THERAPY:
- 1. PRURITUS: This adverse reaction occurs with approximately 10% of all patients. In most cases, pruritus can be alleviated with frequent application of bland emollients or other topical agents; severe pruritus may require systemic treatment. If pruritus is unresponsive to these measures, shield pruritic areas from further UVA exposure until the condition resolves. If intractable pruritus is generalized, UVA treatment should be discontinued until the pruritus disappears.
- 2. ERYTHEMA: Mild, transient erythema at 24-48 hours after PUVA therapy is an expected reaction and indicates that a therapeutic interaction between methoxsalen and UVA occurred. Any area showing moderate erythema (greater than Grade 2; see Table 1 for grades of erythema) should be shielded during subsequent UVA exposures until the erythema has resolved. Erythema greater than Grade 2 which appears within 24 hours after UVA treatment may signal a potentially severe burn. Erythema may become progressively worse over the next 24 hours, since the peak erythemal reaction characteristically occurs 48 hours or later after methoxsalen ingestion. The patient should be protected from further UVA exposures and sunlight, and should be monitored closely.
- 3. IMPORTANT DIFFERENCES BETWEEN PUVA ERYTHEMA AND SUNBURN: PUVA-induced inflammation differs from sunburn or UVB phototherapy in several ways. The percent transmission of UVB varies between 0% to 34% through skin whereas UVA varies between 1% to 80% transmission; thus, UVA is transmitted to a larger percent through the skin. (Diffey, 198222). The DNA lesions induced by PUVA are very different from UV-induced thymine dimers and may lead to a DNA crosslink. This DNA lesion may be more problematic to the cell because crosslinks are more lethal and psoralen-DNA photoproducts may be “new” or unfamiliar substrates for DNA repair enzymes. DNA synthesis is also suppressed longer after PUVA. The time course of delayed erythema is different with PUVA and may not involve the usual mediators seen in sunburn. PUVA-induced redness may be just beginning at 24 hours, when UVB erythema has already passed its peak. The erythema dose-response curve is also steeper for PUVA. Compared to equally erythemogenic doses of UVB, the histologic alterations induced by PUVA show more dermal vessel damage and longer duration of epidermal and dermal abnormalities.
- 4. OTHER ADVERSE REACTIONS: Those reported include edema, dizziness, headache, malaise, depression, hypopigmentation, vesiculation and bullae formation, non-specific rash, herpes simplex, miliaria, urticaria, folliculitis, gastrointestinal disturbances, cutaneous tenderness, leg cramps, hypotension, and extension of psoriasis.
- To report SUSPECTED ADVERSE REACTIONS, contact Valeant Pharmaceuticals North America LLC at 1-800-321-4576 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
- VIII. OVERDOSAGE
-
IX. DRUG DOSAGE AND ADMINISTRATION
CAUTION: Methoxsalen Capsules, USP represents a new dose form of methoxsalen. This new dosage form of methoxsalen exhibits significantly greater bioavailability and earlier photosensitization onset time than previous methoxsalen dosage forms. Each patient should be evaluated by determining the minimum phototoxic dose (MPD) and phototoxic peak time after drug administration prior to onset of photochemotherapy with this dosage form. Human bioavailability studies have indicated the following drug dosage and administration directions are to be used as a guideline only.
PSORIASIS THERAPY
1. DRUG DOSAGE - INITIAL THERAPY: Methoxsalen capsules should be taken 1-1/2 to 2 hours before UVA exposure with some low-fat food or milk according to the following table:
Patient’s Weight
Dose(kg)
(lbs)
(mg)
<30
<66
10
30-50
66-110
20
51-65
112-143
30
66-80
146-176
40
81-90
179-198
50
91-115
201-254
60
>115
>254
70
Geriatric patients should generally be started at the low end of the dose recommended according to body weight and closely monitored during PUVA therapy. Although clinical experience has not identified differences in response between elderly and younger patients, the use of methoxsalen in older individuals may be affected by the presence or pre-existing medical conditions.
2. INITIAL EXPOSURE: The initial UVA exposure energy level and corresponding time of exposure is determined by the patient’s skin characteristics for sun burning and tanning as follows:
*Patients with natural pigmentation of these types should be classified into a lower skin type category if the sunburning history so indicates.
Skin Type
HistoryRecommended
J/cm2I
Always burn, never tan (patients with
erythrodermic psoriasis are to be
classed as Type I for determination
of UVA dosage.)0.5 J/cm2
II
Always burn, but sometimes tan
1.0 J/cm2
III
Sometimes burn, but always tan
1.5 J/cm2
IV
Never burn, always tan
2.0 J/cm2
Skin Type
Physician Examination
J/cm2
V*
Moderately pigmented
2.5 J/cm2
VI*
Blacks
3.0 J/cm2
If the MPD is done, start at 1/2 MPD.
Additional drug dosage directions are as follows:
- a. Weight Change: In the event that the weight of a patient changes during treatment such that he/she falls into an adjacent weight range/dose category, no change in the dose of methoxsalen is usually required. If, in the physician’s opinion, however, a weight change is sufficiently great to modify the drug dose, then an adjustment in the time of exposure to UVA should be made.
- b. Dose/Week: The number of doses per week of methoxsalen capsules will be determined by the patient’s schedule of UVA exposures. In no case should treatments be given more often than once every other day because the full extent of phototoxic reactions may not be evident until 48 hours after each exposure.
- c. Dosage Increase: Dosage may be increased by 10 mg after the 15th treatment under the conditions outlined in section XI.B.4.b.
X. UVA RADIATION SOURCE SPECIFICATIONS AND INFORMATION
A. IRRADIANCE UNIFORMITY:
The following specifications should be met with the window of the detector held in a vertical plane:
- 1. Vertical Variation: For readings taken at any point along the vertical center axis of the chamber (to within 15 cm from the top and bottom), the lowest reading should not be less than 70% of the highest reading.
- 2. Horizontal Variation: Throughout any specific horizontal plane, the lowest reading must be at least 80% of the highest reading, excluding the peripheral 3 cm of the patient treatment space.
B. PATIENT SAFETY FEATURES:
The following safety features should be present: (1) Protection from electrical hazard: All units should be grounded and conform to applicable electrical codes. The patient or operator should not be able to touch any live electrical parts. There should be ground fault protection. (2) Protective shielding of lamps: The patient should not be able to come in contact with the bare lamps. In the event of lamp breakage, the patient should not be exposed to broken lamp components. (3) Hand rails and hand holds: Appropriate supports should be available to the patient. (4) Patient viewing window: A window which blocks UV should be provided for viewing the patient during treatment. (5) Door and latches: Patients should be able to open the door from the inside with only slight pressure to the door. (6) Non-skid floor: The floor should be of a non-skid nature. (7) Thermoregulation: Sufficient air flow should be provided for patient safety and comfort, limiting temperature within the UVA radiator cabinet to approximately less than 100° F. (8) Timer: The irradiator should be equipped with an automatic timer which terminates the exposure at the conclusion of a pre-set time interval. (9) Patient alarm device: An alarm device within the UVA irradiator chamber should be accessible to the patient for emergency activation. (10) Danger label: The unit should have a label prominently displayed which reads as follows:
DANGER - Ultraviolet Radiation - Follow your physician’s instructions - Failure to use protective eyewear may result in eye injury.
C. UVA EXPOSURE DOSIMETRY MEASUREMENTS:
The maximum radiant exposure or irradiance (within ± 15%) of UVA (320-400 nm) delivered to the patient should be determined by using an appropriate radiometer calibrated to be read in J/cm2 or mW/cm2. In the absence of a standard measuring technique approved by the National Bureau of Standards, the system should use a detector corrected to a cosine spatial response. The use and recalibration frequency of such a radiometer for a specific UVA irradiator chamber should be specified by the manufacturer because the UVA dose (exposure) is determined by the design of the irradiator, the number of lamps, and the age of the lamp. If irradiance is measured, the radiometer reading in mW/cm2 is used to calculate the exposure time in minutes to deliver the required UVA in J/cm2 to a patient in the UVA irradiator cabinet. The equation is:
Overexposure due to human error should be minimized by using an accurate automatic timing device, which is set by the operator and controlled by energizing and de-energizing the UVA irradiator lamp. The timing device calibration interval should be specified by the manufacturer. Safety systems should be included to minimize the possibility of delivering a UVA exposure which exceeds the prescribed dose, in the event the timer or radiometer should malfunction.
D. UVA SPECTRAL OUTPUT DISTRIBUTION:
The spectral distributions of the lamps should meet the following specifications:
1As a percentage of total irradiance between 320 and 400 nanometers. Wavelength band (nanometers)
Output1
<310
....................................................................................................
<1
310 to 320
....................................................................................................
1 to 3
320 to 330
....................................................................................................
4 to 8
330 to 340
....................................................................................................
11 to 17
340 to 350
....................................................................................................
18 to 25
350 to 360
....................................................................................................
19 to 28
360 to 370
....................................................................................................
15 to 23
370 to 380
....................................................................................................
8 to 12
380 to 390
....................................................................................................
3 to 7
390 to 400
....................................................................................................
1 to 3
XI. PUVA TREATMENT PROTOCOL
INTRODUCTION:
The Methoxsalen Capsules, USP reach their maximum bioavailability in 1-1/2 to 2 hours after ingestion.
On average, the serum level achieved with Methoxsalen Capsules, USP is twice that obtained with 8-MOP (formerly Oxsoralen) and reach their peak concentration in less than half the time of 8-MOP capsules.
As a result the mean MED J/cm2 for the Methoxsalen Capsules, USP is substantially less than that required for 8-MOP (Levins et al., 1984 and private communication1).
Photosensitivity studies demonstrate a shorter time of peak photosensitivity of 1.5 to 2.1 hours vs. 3.9 to 4.25 hours for regular methoxsalen capsules.
A. INITIAL EXPOSURE: The initial UVA exposures should be conducted according to the guidelines presented previously under IX., Psoriasis Therapy, Drug Dosage-Initial Therapy and Initial Exposure.
B. CLEARING PHASE: Specific recommendations for patient treatment are as follows:
-
1. SKIN TYPES I, II, & III. Patients with skin types I, II, and III may be treated 2 or 3 times per week. UVA exposure may be held constant or increased by up to 1.0 J/cm2 at each treatment, according to the patient’s response. If erythema occurs, however, do not increase exposure time until erythema resolves. The severity and extent of the patient’s erythema may be used to determine whether the next exposure should be shortened, omitted, or maintained at the previous dosage.
(See ADVERSE REACTIONS). - 2. SKIN TYPES IV, V, & VI. Patients with skin types IV, V, and VI may be treated 2 or 3 times per week. UVA exposure may be held constant or increased by up to 1.5 J/cm2 at each treatment unless erythema occurs. If erythema occurs, follow instructions outlined above in the procedures for patients with skin types I, II, and III.
- 3. ERYTHRODERMIC PSORIASIS. Patients with erythrodermic psoriasis should be treated with special attention because pre-existing erythema may obscure observations of possible treatment-related phototoxic erythema. These patients may be treated 2 or 3 times per week, as a Type I patient.
- 4. MISCELLANEOUS SITUATIONS:
- a. If there is no response after a total of 10 treatments, the exposure of UVA energy may be increased by an additional 0.5-1.0 J/cm2 above the prior incremental increases for each treatment. (Example: a patient whose exposure dosage is being increased by 1.0 J/cm2 may now have all subsequent doses increased by 1.5-2.0 J/cm2.)
- b. If there is no response, or only minimal response, after 15 treatments, the dosage of methoxsalen may be increased by 10 mg (a one-time increase in dosage). This increased dosage may be continued for the remainder of the course of treatment but should not be exceeded.
- c. If a patient misses a treatment, the UVA exposure time of the next treatment should not be increased. If more than one treatment is missed, reduce the exposure by 0.5 J/cm2 for each treatment missed.
- d. If the lower extremities are not responding as well as the rest of the body and do not show erythema, cover all other body areas and give 25% of the present exposure dose as an additional exposure to the lower extremities. This additional exposure to the lower extremities should be terminated if erythema develops on these areas.
- e. Non-responsive psoriasis: If a patient’s generalized psoriasis is not responding, or if the condition appears to be worsening during treatment, the possibility of a generalized phototoxic reaction should be considered. This may be confirmed by the improvement of the condition following temporary discontinuance of this therapy for two weeks. If no improvement occurs during the interruption of treatment, this patient may be considered a treatment failure.
C. ALTERNATIVE EXPOSURE SCHEDULE:
As an alternative to increasing the UVA exposure at each treatment, the following schedule may be followed; this schedule may reduce the total number of J/cm2 received by the patient over the entire course of therapy.
- 1. Incremental increases in UVA exposure for all patients may range from 0.5 to 1.5 J/cm2, according to the patient’s response to therapy.
- 2. Once Grade 2 clearing (see Table 2) has been reached and the patient is progressing adequately, UVA dosage is held constant. This dosage is maintained until Grade 4 clearing is reached.
- 3. If the rate of clearing significantly decreases, exposure dosage may be increased at each treatment (0.1-1.5 J/cm2) until Grade 3 clearing and a satisfactory progress rate is attained. The UVA exposure will be held constant again until Grade 4 clearing is attained. These increases may be used also if the rate of clearing significantly decreases between Grade 3 and Grade 4 response. However, the possibility of a phototoxic reaction should be considered; see Non-responsive Psoriasis, above.
- 4. In summary, this schedule raises slightly the increments (J/cm2) of UVA dosage, but limits these increases to those periods when the patient is not responding adequately. Otherwise, the UVA exposure is held at the lowest effective dose.
D. MAINTENANCE PHASE:
The goal of maintenance treatment is to keep the patient as symptom-free as possible with the least amount of UVA exposure.
-
1. SCHEDULE OF EXPOSURES: When patients have achieved 95% clearing, or Grade 4 response (Table 2), they may be placed on the following maintenance schedules (M1 - M4), in sequence. It is recommended that each maintenance schedule be adhered to for at least two treatments (unless erythema or psoriatic flare occurs, in which case see (2a) and (2b) below).
Maintenance Schedules
M1 - once/week
M2 - once/2 weeks
M3 - once/3 weeks
M4 - p.r.n. (i.e., for flares)
-
2. LENGTH OF EXPOSURE: The UVA exposure for the first maintenance treatment of any schedule (except M4 as noted below) is the same as that of the patient’s last treatment under the previous schedule. For skin types I-IV, however, it is recommended that the maximum UVA dosage during maintenance treatments not exceed the following:
Skin Types
J/cm2/treatment
I
12
II
14
III
18
IV
22
-
If the patient develops erythema or new lesions of psoriasis, proceed as follows: a. Erythema: During maintenance therapy, the patient’s tan and threshold dose for erythema may gradually decrease. If maintenance treatments produce significant erythema, the exposure to UVA should be decreased by 25% until further treatments no longer produce erythema.
b. Psoriasis: If the patient develops new areas of psoriasis during maintenance therapy (but still is classified as having a Grade 4 response), the exposure to UVA may be increased by 0.5-1.5 J/cm2 at each treatment; this is appropriate for all types of patients. These increases are continued until the psoriasis is brought under control and the patient is again clear. The exposure being administered when this clearing is reached should be used for further maintenance treatment. - 3. FLARES DURING MAINTENANCE: If the patient flares during maintenance treatment (i.e., develops psoriasis on more than 5% of the originally involved areas of the body), this maintenance treatment schedule may be changed to the preceding maintenance or clearing schedule. The patient may be kept on this schedule until again 95% clear. If the original maintenance treatment schedule is unable to control the psoriasis, the schedule may be changed to a more frequent regimen. If a flare occurs less than 6 weeks after the last treatment, 25% of the maximum exposure received during the clearing phase, with the clearing schedule received during the clearing phase, may be used and then proceed with the clearing schedule previously followed for this patient. (At 95% clearing, follow regular maintenance until the optimum maintenance schedule is determined for the patient.) If more than 6 weeks have elapsed since the last treatment was given, treat patients as if they were beginning therapy insofar as exposure dosages are concerned, since their threshold for erythema may have decreased.
Table 1. Grades of Erythema Grade
Erythema
0
No erythema
1
Minimally perceptible erythema – faint pink
2
Marked erythema but with no edema
3
Fiery erythema with edema
4
Fiery erythema with edema and blistering
Table 2. Response To Therapy
Grade
CriteriaPercent Improvement
(compared to original
extent of disease)-1
Psoriasis worse. . . . . . . . . . . . . . . . . . . . . . . . . . . . .
0
0
No change. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
0
1
Minimal improvement – slightly less scale
and/or erythema. . . . . . . . . . . . . . . . . . . . . . . . . . . .
5-202
Definite improvement – partial flattening
of all plaques – less scaling and less
erythema. . . . . . . . . . . . . . . . . .. . . . . . . . . . . . . . . .
20-503
Considerable improvement – nearly complete
flattening of all plaques but borders
of plaques still palpable. . . . . . . . . . . . . . . . . . . . . . .
50-954
Clearing; complete flattening of plaques
including borders; plaques may be outlined
by pigmentation. . . . . . . . . . . . . . . . . . . . . . . . . . . . .
95 -
XII. HOW SUPPLIED
Methoxsalen Capsules, USP, each containing 10 mg of methoxsalen (8-methoxypsoralen) are available in green soft gelatin capsules in white plastic bottles of 50 (NDC: 68682-065-10), with VRX imprinted on one side of the capsule and 650 imprinted on the other side.
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F).
-
BIBLIOGRAPHY
- 1. Levins, P.C., Gange, R.W., Momtaz-T,K., Parrish, J.A., and Fitzpatrick, T.B.: A New Liquid Formulation of 8-Methoxypsoralen: Bioactivity and Effect of Diet: JID, 82, No. 2, pp. 185-187 (1984) and private communication.
- 2. Artuc,M., Stuettgen, G., Schalla, W., Schaefer, H., and Gazith, J.: Reversible binding of 5-and 8-methoxypsoralen to human serum proteins (albumin) and to epidermis in vitro: Brit. J. Dermat. 101, pp. 669-677 (1979).
- 3. Mandula, B.B., Pathak, M.A., Nakayama, T., and Davidson, S.J.: Induction of mixed-function oxidases in mouse liver by psoralens., Ibid, 99, pp. 687-692 (1978).
- 4. Pathak, M.A., Fitzpatrick, T.B., Parrish, J.A.: PSORIASIS, Proceedings of the Second International Symposium. Edited by E.M. Farber, A.J. Cox, Yorke Medical Books, pp. 262-265 (1977).
- 5. Dall’Acqua, F., Marciani, S., Ciavatta, L., Rodighiero, G.: Formation of interstrand cross-linkings in the photoreactions between furocoumarins and DNA; Z Naturforsch (B), 26, pp. 561-569 (1971).
- 6. Cole, R.S.: Light-induced cross-linkings of DNA in the presence of a furocoumarin (psoralen), Biochem. Biophys. Acta, 217, pp. 30-39 (1970).
- 7. Musajo, L., Rodighiero, G., Caporale, G., Dall’Acqua, F., Marciani, S., Bordin, F., Baccichetti, F., Bevilacqua, R.: Photoreactions between Skin-Photosensitizing Furocoumarins and Nucleic Acids, Sunlight and Man; Normal and Abnormal Photobiologic Responses. Edited by M.A. Pathak, L.C. Harber, M. Seiji et al. University of Tokyo Press, pp. 369-387 (1974).
- 8. Dall’Acqua, F., Vedaldi, D., Bordin, F., and Rodighiero, G.: New studies in the interaction between 8-methoxypsoralen and DNA in vitro; JID, 73, pp. 191-197 (1979).
- 9. Yoshikawa, K., Mori, N., Sakakibara, S., Mizuno, N., Song, P.: Photo Conjugation of 8-methoxypsoralen with Proteins; Photochem. & Photobiol. 29, pp. 1127-1133 (1979).
- 10. Hakim, R.D., Griffin, A.C.: Knox, J.M.: Erythema and tumor formation in methoxsalen treated mice exposed to fluorescent light; Arch. Dermatol. 82, pp. 572-577 (1960).
- 11. O’Neal, M.A., Griffin, A.C.: The Effect of Oxypsoralen upon Ultraviolet Carcinogenesis in Albino Mice, Cancer Res., 17, pp. 911-916 (1957).
- 12. Stern, R.S., Unpublished personal communication.
- 13. Stern, R.S., Parrish, J.A., Zierler, S.: Skin Carcinoma in Patients with Psoriasis Treated with Topical Tar and Artificial Ultraviolet Radiation. Lancet, 1, pp. 732-735 (1980).
- 14. Stern, R.S., Laird, N., Melski, J., Parrish, J.A., Fitzpatrick, T.B., Bleich, H.L.: Cutaneous Squamous-Cell Carcinoma in Patients Treated with PUVA: NEJM, 310, No. 18, pp. 1156-1161 (1984).
- 15. Roenigk, Jr., H.H., and 12 Cooperating Investigators: Skin Cancer in the PUVA-48 Cooperative Study of Psoriasis. Program for Forty-First Annual Meeting for The Society of Investigative Dermatology, Inc., Sheraton Washington Hotel, Washington, D.C., May 12, 13, and 14, 1980. Abstracts JID, 74, No. 4, p. 250 (April, 1980).
- 16. Stern et al., Malignant melanoma in patients treated for psoriasis with methoxsalen (psoralen) and ultraviolet A radiation (PUVA). The PUVA Follow-up Study. New England Journal of Medicine, 336:1041-1045, (April 10, 1997).
- 17. Mosher, D.B., Pathak, M.A., Harris, T.J., Fitzpatrick, T.B.: Development of Cutaneous Lesions in Vitiligo During Long-Term PUVA Therapy. Program for Forty-First Annual Meeting for The Society for Investigative Dermatology, Inc., Sheraton Washington Hotel, Washington, D.C., May 12, 13, and 14, 1980. Abstracts JID, 74, No. 4, p. 259 (April, 1980).
- 18. Cloud, T.M., Hakim, R., Griffin, A.C.: Photosensitization of the eye with methoxsalen. I. Acute effects; Arch. Ophthalmol. 64, pp. 346-352 (1960).
- 19. Cloud, T.M., Hakim, R., Griffin, A.C.: Photosensitization of the eye with methoxsalen. II. Chronic effects, Ibid, 66, pp. 689-694 (1961).
- 20. Freeman, R.G., Troll, D.: Photosensitization of the eye by 8-methoxypsoralen, JID, 53, pp. 449-453 (1969).
- 21. Lerman, S., Megaw, J., Willis, I.: Potential ocular complications from PUVA therapy and their prevention; JID 74, pp. 197-199 (1980).
- 22. Diffey, B.L., Medical Physics Handbook 11, Ultraviolet Radiation In Medicine, Adam Hilger, Ltd., Bristol, p. 86 (1982).
-
SPL UNCLASSIFIED SECTION
Manufactured for:
Oceanside Pharmaceuticals, a division of
Valeant Pharmaceuticals North America LLC
Bridgewater, NJ 08807 USABy:
Heritage Pharma Labs, Inc.
East Brunswick, NJ 08816 USA®/™ are trademarks of Valeant Pharmaceuticals International, Inc. or its affiliates.
© 2018 Valeant Pharmaceuticals North America LLCRev. 06/18
9647500
70013293 -
PATIENT INFORMATION ON THE USE OF METHOXSALEN CAPSULES, USP 10 mg IN THE TREATMENT OF PSORIASIS
- This brochure is intended to provide you with information about the treatment of psoriasis. The entire brochure should be read so that you are aware of the requirements on your part to ensure the effectiveness and safety of the therapy. Any additional questions that you may have can be answered by your doctor or pharmacist. In addition, the pharmacist will have a copy of a very technical brochure entitled the “Physician’s Package Insert” that you may wish to read.
- A. What Is Methoxsalen Capsules, USP?
- Methoxsalen Capsules, USP is a drug which has been shown to be effective in the treatment of psoriasis when combined with exposure to a very specific kind of light. The use of the drug must be combined with exposure to the special light to produce effective therapy.
- Methoxsalen Capsules, USP represents a new dose form of methoxsalen. This new dosage form of methoxsalen exhibits significantly greater bioavailability and earlier photosensitization onset time than previous methoxsalen dosage forms.
- B. What Is The Special Light?
- Light is classified into many different parts. One part is known as ultraviolet light, which is a normal component of sunlight. Artificial or man-made light sources are now available that produce the special part of light (ultraviolet “A”) necessary for the most effective therapy.
- C. What Is “PUVA”?
- “PUVA” is the name of the treatment for psoriasis and stands for the use of Psoralen drug Methoxsalen Capsules, USP in combination with UltraViolet A light.
- D. What Is Psoriasis?
- Psoriasis is a skin condition associated with red and scaly patches. The cause of psoriasis is not known. PUVA (Methoxsalen Capsules, USP with ultraviolet A light) is used for the treatment of severe psoriasis that has not been helped by other methods of therapy.
- E. What Should The Patient Do Before PUVA Therapy?
-
Certain other medicines can make you more sensitive to the combination drug and light treatment. In addition, certain other medical conditions can be aggravated by this treatment. Before starting treatment, be sure to tell your doctor if you have experienced any of the following:
- 1) had a severe reaction to Methoxsalen Capsules, USP in the past
- 2) had recent x-ray treatment or planning any
- 3) have or ever have had skin cancer
- 4) have or ever have had any eye problems such as cataracts or loss of the lens of the eyes
- 5) have or ever have had liver problems
- 6) have or ever have had heart or blood pressure problems
- 7) have any medical condition that requires you to stay out of the sun such as lupus erythematosus
- 8) are taking any drugs (either prescription or nonprescription). Some drugs can increase your sensitivity to ultraviolet light either from the sun or man-made sources. Examples of such drugs include major tranquilizers, sulfa drugs for the treatment of infection or diabetes, tetracycline, antibiotics, griseofulvin products, thiazide-containing diuretics (blood pressure or water elimination drugs), and certain antibacterial or deodorant soaps.
- F.
How Should The Patient Take Methoxsalen Capsules, USP?
- 1) The number of capsules recommended by your doctor should be taken with some food or low-fat milk 2 hours before ultraviolet light treatment.
- 2) Methoxsalen Capsules, USP is a potent drug. Never take more than is prescribed for you since it may result in burning and/or blistering of your skin after exposure to ultraviolet light.
- G.
What Precautions Should Be Taken During And After PUVA Therapy?
- 1) Eye Protection – Make sure that you wear special wrap-around sunglasses that totally block or absorb ultraviolet light. Put them on immediately after taking Methoxsalen Capsules, USP and continue wearing them for 24 hours if any light is present (even if indirect such as reflection or through window glass). Ordinary sunglasses are not adequate.
- 2) Skin & Lip Protection – Do not allow exposure of your skin and lips to sunlight for 8 hours after treatment. In addition, do not expose your skin to either sunlight or sun lamps (regardless of safety claims) within 24 hours of a scheduled treatment. It is advisable to wear protective clothing (hat, gloves) to cover as much of your body as possible after treatment as well as using a sun screen product having a protection factor of at least 15 (only use after treatment).
- H. How Long Will The Treatments Last?
- May take from six to eight weeks before lesions disappear. Maintenance treatments are usually needed to keep the disease under control.
- I.
What Are The Problems Associated With Pregnancy Or Breast Feeding?
- 1) Birth control methods should be employed since the effects of PUVA therapy on the unborn child are not known. If you become pregnant, inform your doctor so that he can determine whether it is necessary for you to temporarily stop therapy.
- 2) Since it is not known whether Methoxsalen Capsules, USP passes into mother’s milk, it is safer not to breast feed while taking this drug.
- J.
What Are The Risks Of PUVA Therapy?
- 1) Premature skin aging may result from prolonged PUVA therapy, especially with those individuals who tan poorly. This problem is similar to excessive exposure to sunlight.
- 2) There is an increased risk of developing both melanoma and non-melanoma skin cancer. This risk is greater for individuals who fall into the following categories:
- a) fair skin that burns rather than tans
- b) have had prior treatment with x-rays, grenz rays, or arsenic
- c) have had coal tar and UltraViolet B (UVB treatment).
- Even though your doctor will be examining you, you should routinely and completely examine yourself for small growths on your skin or skin sores that will not heal. Immediately report such observations to your doctor.
- 3) Since studies have shown that animals with unprotected eyes have developed cataracts after PUVA therapy, you should have your eyes examined by an ophthalmologist before starting PUVA therapy, after the first year of therapy and every two years thereafter.
- K.
What Are The Possible Side Effects?
- 1) The most common side effects of PUVA therapy are nausea, itching, and redness of the skin. The use of low-fat milk or food when ingesting the drug may prevent the nausea.
- 2) Tenderness or blistering of the skin may occur, but these symptoms can be helped by the use of skin products recommended by your doctor or pharmacist.
- 3) Less frequent side effects include depression, dizziness, headache, swelling, rash or leg cramps. Important: Contact your doctor if any side effect continues to bother you after 24-48 hours.
- L.
What Else Should The Patient Know?
- 1) Remember to take Methoxsalen Capsules, USP as directed by your doctor. If you forget to take the drug before your scheduled treatment, be sure to call your doctor to determine what he wishes you to do.
- 2) Remember that the drug has been prescribed specifically for you and your diagnosed condition. Do not use the drug for any other conditions nor give the drug to others even if they have similar symptoms.
- 3) If you think that you or anyone else has accidentally taken an overdose, stay out of the sunlight and immediately contact your poison control center, doctor, pharmacist, or nearest hospital emergency room.
- 4) ALWAYS KEEP THIS DRUG AND ALL OTHER DRUGS OUT OF THE REACH OF CHILDREN.
- 5) Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F).
Manufactured for:
Oceanside Pharmaceuticals, a division of
Valeant Pharmaceuticals North America LLC
Bridgewater, NJ 08807 USABy:
Heritage Pharma Labs, Inc.
East Brunswick, NJ 08816 USA®/™ are trademarks of Valeant Pharmaceuticals International, Inc. or its affiliates.
© 2018 Valeant Pharmaceuticals North America LLCRev. 06/18
9647500
70013293 -
PRINCIPAL DISPLAY PANEL - 10 mg Bottle Label
NDC 68682-065-10
Rx onlyMETHOXSALEN
CAPSULES, USP10 mg
CAUTION: This new dosage form of methoxsalen exhibits significantly greater bioavailabilty and earlier photosensitization onset time than previous methoxsalen dosage forms. Each patient should be evaluated by determining the minimum phototoxic dose (MPD) and phototoxic peak time after drug administration prior to onset of photochemotherapy with this dosage form.
50 Capsules
OCEANSIDE
PHARMACEUTICALS -
INGREDIENTS AND APPEARANCE
METHOXSALEN
methoxsalen capsule, liquid filledProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68682-065 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHOXSALEN (UNII: U4VJ29L7BQ) (METHOXSALEN - UNII:U4VJ29L7BQ) METHOXSALEN 10 mg Inactive Ingredients Ingredient Name Strength D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color GREEN Score no score Shape CAPSULE Size 19mm Flavor Imprint Code VRX;650 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68682-065-10 50 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/15/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA authorized generic NDA019600 07/15/2014 Labeler - Oceanside Pharmaceuticals (832011691) Establishment Name Address ID/FEI Business Operations HERITAGE PHARMA LABS, INC 189630168 MANUFACTURE(68682-065) , PACK(68682-065) Establishment Name Address ID/FEI Business Operations Carton Service Incorporated 928861723 PACK(68682-065) , LABEL(68682-065) , RELABEL(68682-065) Establishment Name Address ID/FEI Business Operations Aphena Pharma Solutions 128385585 PACK(68682-065) , LABEL(68682-065) , RELABEL(68682-065)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.