VYALEV- foscarbidopa/foslevodopa injection
Vyalev by
Drug Labeling and Warnings
Vyalev by is a Prescription medication manufactured, distributed, or labeled by AbbVie Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use VYALEV safely and effectively. See full prescribing information for VYALEV.
VYALEV™ (foscarbidopa and foslevodopa) injection, for subcutaneous use
Initial U.S. Approval: 1975
INDICATIONS AND USAGE
VYALEV is a combination of foscarbidopa (an aromatic amino acid decarboxylation inhibitor) and foslevodopa (an aromatic amino acid) indicated for the treatment of motor fluctuations in adults with advanced Parkinson’s disease. (1)
DOSAGE AND ADMINISTRATION
- For subcutaneous administration only, preferably in the abdomen, via the VYAFUSER pump. (2.1, 2.2, 2.3)
- See the Full Prescribing Information for calculation of the base continuous dosage, hourly infusion rate, optional loading dose, and extra dose. (2.2)
- The maximum recommended daily dosage of VYALEV is 3,525 mg of foslevodopa (approximately 2,500 mg levodopa). (2.2)
DOSAGE FORMS AND STRENGTHS
Injection: 120 mg foscarbidopa and 2,400 mg foslevodopa per 10 mL (12 mg foscarbidopa and 240 mg foslevodopa per mL). (3)
CONTRAINDICATIONS
VYALEV is contraindicated in patients who are currently taking a nonselective monoamine oxidase (MAO) inhibitor or have recently (within 2 weeks) taken a nonselective MAO inhibitor. (4)
WARNINGS AND PRECAUTIONS
- May cause falling asleep during activities of daily living. (5.1)
- Hallucinations/Psychosis: May respond to dose reduction of VYALEV. (5.2)
- Impulse Control Behaviors: Consider dose reductions or stopping VYALEV. (5.3)
- Infusion Site Reactions and Infections: Monitor for infusion site infections: Following aseptic techniques while using this medication and frequent rotation of the infusion site is recommended to reduce the risk. (5.4)
- Avoid sudden discontinuation or rapid dose reduction to reduce the risk of withdrawal-emergent hyperpyrexia and confusion. (5.5)
- May cause or exacerbate dyskinesia: Consider dose reduction of VYALEV. (5.6)
ADVERSE REACTIONS
Most common adverse reactions for VYALEV (VYALEV incidence at least 10% and greater than oral carbidopa-levodopa incidence) were infusion/catheter site reactions, infusion/catheter site infections, hallucinations, and dyskinesia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact AbbVie Inc. at 1-800-633-9110 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
Pregnancy: Based on animal data, may cause fetal harm. (8.1)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 10/2024
- For subcutaneous administration only, preferably in the abdomen, via the VYAFUSER pump. (2.1, 2.2, 2.3)
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Information
2.2 Recommended Dosage
2.3 Preparation and Administration Instructions
2.4 Interruption of Therapy
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Falling Asleep During Activities of Daily Living and Somnolence
5.2 Hallucinations/Psychosis
5.3 Impulse Control/Compulsive Behaviors
5.4 Infusion Site Reactions and Infections
5.5 Withdrawal-Emergent Hyperpyrexia and Confusion
5.6 Dyskinesia
5.7 Cardiovascular Ischemic Events
5.8 Glaucoma
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Monoamine Oxidase (MAO) Inhibitors
7.2 Antihypertensive Drugs
7.3 Dopamine D2 Receptor Antagonists and Isoniazid
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2
DOSAGE AND ADMINISTRATION
2.1 Important Information
For subcutaneous administration only.
Patients selected for treatment with VYALEV should be capable of understanding and using the delivery system [see Healthcare Professional Instructions for Use of VYAFUSER Pump, and Patient Instructions for Use of VYAFUSER Pump] themselves or with assistance from a caregiver.
Patients should be trained on the proper use of VYALEV and the delivery system prior to initiating.
2.2 Recommended Dosage
VYALEV (foscarbidopa and foslevodopa) is administered as a subcutaneous infusion with the VYAFUSER pump [see Dosage and Administration (2.3), Healthcare Professional Instructions for Use of VYAFUSER Pump, and Patient Instructions for Use of VYAFUSER Pump].
VYALEV Base Continuous Dosage and Hourly Infusion Rate
The continuous infusion rate is based on total levodopa dosage (TLD). The hourly base continuous infusion rate (mL/hr) = [(TLD x 1.3) / 240] / [number of hours the patient is typically awake (e.g., 16 hours)], as shown in the steps below.
Step 1: Calculate the TLD for the levodopa-containing medications that VYALEV is replacing. All dosages should be converted to the equivalent dosage of immediate-release levodopa to obtain the TLD. Prescribers should adjust the total dose of levodopa-containing products for COMT inhibitor use. See the Prescribing Information for the respective drugs for conversions or adjustments. Do not include rescue or as needed levodopa or any other anti-Parkinsonian medication or therapy, including medications taken outside of awake time (e.g., night-time dosing) in this calculation.
Step 2: Determine the total daily dosage (mg) of VYALEV foslevodopa component by multiplying the TLD by 1.3. The conversion factor takes into account the molecular weight and bioavailability of foslevodopa compared to levodopa.
Step 3: Determine the total daily volume (mL) of VYALEV by dividing the total daily dosage (mg) of VYALEV by 240. Each 1 mL of VYALEV contains 240 mg of foslevodopa.
Step 4: Determine the hourly base continuous infusion rate of VYALEV by dividing the total daily volume (mL) of VYALEV by the number of hours the patient is typically awake (e.g., 16 hours). VYALEV may be administered over the patient’s waking hours or may be administered for 24 hours. See Dosage and Administration (2.3) for adjustment of and alternate infusion rates for lower overnight dosages.
Maximum Dosage
The maximum recommended daily dosage of VYALEV is 3,525 mg of the foslevodopa component (equivalent to approximately 2,500 mg levodopa).
Optional Loading Dose
If VYALEV therapy is being initiated in an “Off” state or the patient has not been receiving their base continuous infusion for more than 3 hours, a loading dose can be administered immediately prior to starting or re-starting the base continuous hourly infusion. Loading doses can be administered either with VYALEV or patients can continue using oral immediate-release carbidopa/levodopa tablets. The loading dose should be calculated from the first morning dose of oral immediate release carbidopa/levodopa the patient took before starting treatment with VYALEV. If VYALEV is used for the loading dose, multiply the first morning dose of oral immediate release levodopa by 1.3 and divide by 240 to determine the loading dose of VYALEV.
The pump is capable of delivering a loading dose ranging from 0.1 mL to a maximum of 3 mL, in increments of 0.1 mL.
Extra Dose
An extra dose volume can be programmed to 1 of 5 options (see Table 1). The “extra dose” feature is limited to no more than 1 extra dose per hour. If 2 or more extra doses are used by the patient during the 24-hour/day treatment period, a revision of the base continuous infusion rate should be considered (refer to the Healthcare Professional Instructions for Use of VYAFUSER Pump details).
Table 1. Extra Dose Option for VYALEV VYALEV extra dose volume Approximate equivalent levodopa dose* 0.1 mL 17 mg 0.15 mL 25.5 mg 0.2 mL 34 mg 0.25 mL 42.5 mg 0.3 mL 51 mg *See Step 1
2.3 Preparation and Administration Instructions
Patients should be trained on the proper use of VYALEV and the delivery system prior to initiating treatment with VYALEV and, as necessary, thereafter [see Dosage and Administration (2.2) and the Patient Instructions for Use of VYAFUSER Pump].
Preparation
- Use aseptic technique.
- Use sterile, single-patient-use infusion components (syringe, infusion set, and vial adapter) qualified for use with the pump to infuse VYALEV.
- Do not dilute.
- Do not mix VYALEV with other products.
- The medication vials are for single dose only. The entire contents of a VYALEV vial should be transferred into a syringe for administration. Do not withdraw a partial portion of the vial contents.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit [see Dosage Forms and Strengths (3)].
- The infusion set (cannula) can remain in place for up to 3 days when VYALEV is infused continuously.
Administration
Site Selection
- Administer VYALEV subcutaneously, preferably in the abdomen, avoiding the area with a 2-inch radius from the navel.
- Rotate the infusion site and use a new infusion set at least every 3 days.
- Select new infusion sites at least 1 inch from sites used within the previous 12 days.
- Do not infuse VYALEV into areas where the site is tender, bruised, red, or hard to the touch.
Infusion Flow Rates
- Adjustment: Infusion rates may be adjusted in increments of 0.01 mL/hr (which is equivalent to approximately 1.7 mg of levodopa/hour).
- Alternative Flow Rates: The healthcare professional has the option to enable and pre-program the pump with 2 alternative continuous infusion rates (Low/High) that the patient may select to account for changes in functional demand (e.g., lowering the dosage at night or increasing the dose for prolonged intense activity) (see the Healthcare Professional Instructions for Use of VYAFUSER Pump).
Discarding VYALEV and Syringe
- Discard the vial after transfer of the product to the syringe. Discard the syringe and any unused VYALEV in the syringe after the product has been in the syringe for 24 hours.
2.4 Interruption of Therapy
Prescribing a backup oral carbidopa and levodopa product is recommended in the event that delivery of VYALEV is interrupted, which may result in underdosing. Sudden discontinuation or rapid dose reduction of VYALEV, without administration of alternative dopaminergic therapy, should be generally avoided [see Warnings and Precautions (5.5)].
Following interruptions of more than 1 hour, a new infusion set (tubing and cannula) should be used and rotated to a different infusion site. If the infusion has been interrupted for longer than 3 hours, the patient may also self-administer a loading dose, if enabled by their healthcare professional [see Dosage and Administration (2.2)].
- Use aseptic technique.
- 3 DOSAGE FORMS AND STRENGTHS
-
4
CONTRAINDICATIONS
VYALEV is contraindicated in patients who are currently taking a non-selective monoamine oxidase (MAO) inhibitor or have recently (within 2 weeks) taken a nonselective MAO inhibitor. Hypertension can occur if these drugs are used concurrently [see Drug Interactions (7.1)].
-
5
WARNINGS AND PRECAUTIONS
5.1 Falling Asleep During Activities of Daily Living and Somnolence
Patients treated with levodopa (the active metabolite of VYALEV) have reported falling asleep while engaged in activities of daily living, including the operation of motor vehicles, which sometimes resulted in accidents. Although many of these patients reported somnolence while on levodopa, some perceived that they had no warning signs, such as excessive drowsiness, and believed that they were alert immediately prior to the event (sleep attack). Some of these events have been reported more than one year after initiation of treatment.
Falling asleep while engaged in activities of daily living usually occurs in patients experiencing preexisting somnolence, although patients may not give such a history. For this reason, prescribers should reassess patients for drowsiness or sleepiness while using VYALEV, especially since some of the events occur well after the start of treatment. Prescribers should be aware that patients may not acknowledge drowsiness or sleepiness until directly questioned about drowsiness or sleepiness during specific activities. Patients who have already experienced somnolence or an episode of sudden sleep onset should not participate in these activities while taking VYALEV.
Before initiating treatment with VYALEV, advise patients about the potential to develop drowsiness and specifically ask about factors that may increase the risk for somnolence with VYALEV such as the use of concomitant sedating medications or the presence of sleep disorders. Consider discontinuing VYALEV in patients who report significant daytime sleepiness or episodes of falling asleep during activities that require active participation (e.g., conversations, eating). If VYALEV is continued, patients should be advised not to drive and to avoid other potentially dangerous activities that might result in harm if the patient becomes somnolent. There is insufficient information to establish that dose reduction will eliminate episodes of falling asleep while engaged in activities of daily living.
5.2 Hallucinations/Psychosis
There is an increased risk for hallucinations and psychosis in patients taking VYALEV. In Study 1 [see Clinical Studies (14)], hallucinations occurred in 12.2% of patients treated with VYALEV compared to 1.5% of patients treated with oral immediate-release carbidopa-levodopa. Psychosis occurred in 4.1% of patients treated with VYALEV compared to 1.5% of patients treated with oral immediate-release carbidopa-levodopa. Treatment with VYALEV was discontinued in 1 (1.4%) patient because of hallucinations.
Hallucinations associated with levodopa may present shortly after the initiation of therapy and may be responsive to dose reduction of VYALEV or other concomitantly administered medications. Confusion, insomnia, and excessive dreaming may accompany hallucinations. Abnormal thinking and behavior may present with one or more symptoms, including paranoid ideation, delusions, hallucinations, confusion, psychosis, disorientation, aggressive behavior, agitation, and delirium. Review of treatment is recommended if these symptoms develop.
Because of the risk of exacerbating psychosis, patients with a major psychotic disorder should not be treated with VYALEV. In addition, medications that antagonize the effects of dopamine used to treat psychosis may exacerbate the symptoms of PD and may decrease the effectiveness of VYALEV [see Drug Interactions (7.3)].
5.3 Impulse Control/Compulsive Behaviors
Patients may experience intense urges to gamble, increased sexual urges, intense urges to spend money, binge or compulsive eating, and/or other intense urges, and the inability to control these urges while taking one or more of the medications, including VYALEV, that increase central dopaminergic tone and that are generally used for the treatment of PD. In some cases, although not all, these urges were reported to have stopped when the dose was reduced, or the medication was discontinued.
Because patients may not recognize these behaviors as abnormal, it is important for prescribers to ask patients or their caregivers specifically about the development of new or increased gambling urges, sexual urges, uncontrolled spending, binge or compulsive eating, or other urges while being treated with VYALEV. Consider reducing the dose or discontinuing VYALEV if a patient develops such urges.
5.4 Infusion Site Reactions and Infections
VYALEV can cause infusion site reactions and infections.
In Study 1, one or more infusion site reactions were reported in 62% of patients treated with VYALEV and 8% of patients who received placebo subcutaneous infusion. Various types of reactions at the infusion site have been reported including: erythema, pain, edema, nodule, bruising, hemorrhage, induration, pruritus, extravasation, inflammation, mass, warmth, hematoma, pallor, rash, and swelling. In Study 1, 8% of patients treated with VYALEV and no patient who received placebo withdrew from treatment because of an infusion site reaction.
In Study 1, infusion site infections occurred in 28% of patients treated with VYALEV compared to 3% of patients who received placebo subcutaneous infusion. In Study 1, 5% of patients treated with VYALEV and 2% who received placebo withdrew from treatment because of an infusion site infection. The most frequent infusion site infection reported was cellulitis. If an infection is suspected at the infusion site, the cannula should be removed from the infusion site. If the cannula is removed for an infection, either a new canula should be placed at a new infusion site or, in the event of a prolonged interruption, the patient should be prescribed an oral carbidopa and levodopa product until they are able to resume VYALEV [see Dosage and Administration (2.3, 2.4)].
5.5 Withdrawal-Emergent Hyperpyrexia and Confusion
A symptom complex that resembles neuroleptic malignant syndrome (characterized by elevated temperature, muscular rigidity, altered consciousness, and autonomic instability), with no other obvious etiology, has been reported in association with rapid dose reduction, withdrawal of, or changes in dopaminergic therapy. Avoid sudden discontinuation or rapid dose reduction in patients taking VYALEV. If VYALEV is discontinued, the dose should be tapered to reduce the risk of hyperpyrexia and confusion [see Dosage and Administration (2.4)].
5.6 Dyskinesia
VYALEV may cause or exacerbate dyskinesias. In Study 1, dyskinesia occurred in 11% of patients treated with VYALEV compared to 6% of patients treated with oral immediate-release carbidopa-levodopa. The occurrence of dyskinesias may require a dosage reduction of VYALEV or other medications used to treat PD.
5.7 Cardiovascular Ischemic Events
In clinical studies, myocardial infarction and arrhythmia were reported in patients taking carbidopa-levodopa (the active metabolites of VYALEV). Ask patients about symptoms of ischemic heart disease and arrhythmia, especially those with a history of myocardial infarction or cardiac arrhythmias.
-
6
ADVERSE REACTIONS
The following serious adverse reactions are discussed below and elsewhere in labeling:
- Falling Asleep During Activities of Daily Living and Somnolence [see Warnings and Precautions (5.1)]
- Hallucinations/Psychosis [see Warnings and Precautions (5.2)]
- Impulse Control/Compulsive Behaviors [see Warnings and Precautions (5.3)]
- Infusion Site Reactions and Infections [see Warnings and Precautions (5.4)]
- Withdrawal-Emergent Hyperpyrexia and Confusion [see Warnings and Precautions (5.5)]
- Dyskinesia [see Warnings and Precautions (5.6)]
- Cardiovascular Ischemic Events [see Warnings and Precautions (5.7)]
- Glaucoma [see Warnings and Precautions (5.8)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
In Study 1, a 12-week, active-controlled clinical trial, a total of 141 patients with advanced PD were enrolled [see Clinical Studies (14)]. Of these, 74 patients received VYALEV and 67 received oral immediate-release carbidopa-levodopa with placebo subcutaneous infusion.
Adverse reactions led to discontinuation of VYALEV in 22% of patients, which included hallucinations, infusion site reactions, and infusion site infections [see Warnings and Precautions (5.2, 5.5)]. Table 2 presents the adverse reactions that occurred in ≥3% of patients who received VYALEV and with a difference of >2% between the VYALEV and the oral immediate release carbidopa-levodopa groups in Study 1.
Table 2. Adverse Reactions in Study 1 that Occurred in ≥3% of Patients with Advanced PD who Received VYALEV and 2% Difference from Active Control Adverse Reaction VYALEV
(n = 74)
%Oral immediate-release carbidopa-levodopa
(n = 67)
%Infusion/catheter site reactiona 62 8 Infusion/catheter site infectionb 28 3 Hallucination 12 2 Dyskinesia 11 6 On and off phenomenon 8 0 Balance disorder 5 0 Constipation 5 0 Peripheral swelling 5 0 Agitation 4 2 Insomnia 4 2 Psychotic disorderc 4 2 Dyspnea 4 0 - Infusion/catheter site reaction includes multiple related terms.
- Infusion site/catheter site infections includes multiple related terms.
- Psychotic disorder includes psychotic disorder, delusion, and paranoia.
- Falling Asleep During Activities of Daily Living and Somnolence [see Warnings and Precautions (5.1)]
-
7
DRUG INTERACTIONS
7.1 Monoamine Oxidase (MAO) Inhibitors
Nonselective MAO Inhibitors
The use of nonselective MAO inhibitors (e.g., phenelzine and tranylcypromine) with VYALEV is contraindicated [see Contraindications (4)]. Discontinue use of any nonselective MAO inhibitors at least two weeks prior to initiating VYALEV.
Selective MAO Inhibitors
The use of selective MAO-B inhibitors (e.g., rasagiline and selegiline) with VYALEV may be associated with orthostatic hypotension. Monitor patients who are taking these drugs.
7.2 Antihypertensive Drugs
The concurrent use of VYALEV with antihypertensive medications can cause symptomatic postural hypotension. A dose reduction of the antihypertensive medication may be needed after starting or increasing the dosage of VYALEV.
7.3 Dopamine D2 Receptor Antagonists and Isoniazid
Dopamine D2 receptor antagonists (e.g., phenothiazines, butyrophenones, risperidone, metoclopramide, papaverine) and isoniazid may reduce the effectiveness of foslevodopa. Monitor patients for worsening Parkinson’s symptoms when patients are taking these medications with VYALEV.
-
8
USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no data on the developmental risk associated with the use of VYALEV (foscarbidopa and foslevodopa) in pregnant women. Foscarbidopa is a prodrug of carbidopa, and foslevodopa is a prodrug of levodopa. In animal studies, carbidopa-levodopa has been shown to be developmentally toxic (including teratogenic effects) at clinically relevant doses (see Data).
The estimated background risk of major birth defects and miscarriage in the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
When administered to pregnant rabbits throughout organogenesis, carbidopa-levodopa caused both visceral and skeletal malformations in fetuses at all doses and ratios of carbidopa-levodopa tested. No teratogenic effects were observed when carbidopa-levodopa was administered to pregnant mice throughout organogenesis. There was a decrease in the number of live pups delivered by rats receiving carbidopa-levodopa during organogenesis.
8.2 Lactation
Risk Summary
Foscarbidopa and Foslevodopa
There are no adequate data on the presence of foscarbidopa or foslevodopa in human milk, the effects of foscarbidopa or foslevodopa on milk production or on the breastfed infant.
Foscarbidopa is a prodrug of carbidopa, and foslevodopa is a prodrug of levodopa.
Carbidopa
There are no adequate data on the presence of carbidopa in human milk, the effects on the breastfed infant, or the effects on milk production. Carbidopa is excreted in rat milk.
Levodopa
Levodopa has been detected in human milk after administration of carbidopa-levodopa. Levodopa decreases secretion of prolactin in humans, which may inhibit lactation. There are no adequate data on the effects of levodopa on the breastfed infant.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for VYALEV and any potential adverse effects on the breastfed infant from VYALEV or from the underlying maternal condition.
8.5 Geriatric Use
Clinical studies of VYALEV did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy.
-
10
OVERDOSAGE
In the event of an overdosage with VYALEV, the infusion should be stopped immediately. Administer intravenous fluids and maintain an adequate airway. Electrocardiographic monitoring should be used, and the patient observed carefully for the development of cardiac arrhythmias; if necessary, an appropriate antiarrhythmic therapy should be given. Patients must also be monitored for hypotension.
-
11
DESCRIPTION
VYALEV injection is a solution that is a combination of foscarbidopa (carbidopa-4´-monophosphate) and foslevodopa (levodopa-4´-monophosphate).
Foscarbidopa and foslevodopa are prodrugs that undergo enzymatic bioconversion via intrinsic alkaline phosphatase to carbidopa and levodopa, respectively, in vivo.
Foscarbidopa, an inhibitor of aromatic amino acid decarboxylation, is a white to light yellow powder, freely soluble in aqueous media, with a molecular weight of 306.21 g/mol anhydrous. It is designated chemically as (2S)-2-hydrazinyl-3-[3-hydroxy-4-(phosphonooxy)phenyl]-2-methylpropanoic acid trihydrate. Its empirical formula is C10H15N2O7P (anhydrous basis), and its structural formula is:
![Foscarbidopa, an inhibitor of aromatic amino acid decarboxylation, is a white to light yellow powder, freely soluble in aqueous media, with a molecular weight of 306.21 g/mol anhydrous. It is designated chemically as (2S)-2-hydrazinyl-3-[3-hydroxy-4-(phosphonooxy)phenyl]-2-methylpropanoic acid trihydrate. Its empirical formula is C10H15N2O7P (anhydrous basis), and its structural formula is:](https://fda.report/DailyMed/28e806e4-951c-40a9-9f0c-d0929caf054c/vyalev-01.jpg)
Foslevodopa, an aromatic amino acid, is a white to off-white powder, freely soluble in aqueous media, with a molecular weight of 277.17 g/mol. It is designated chemically as (2S)-2-amino-3-[3-hydroxy-4-(phosphonooxy)phenyl] propanoic acid. Its empirical formula is C9H12NO7P, and its structural formula is:
![Foslevodopa, an aromatic amino acid, is a white to off-white powder, freely soluble in aqueous media, with a molecular weight of 277.17 g/mol. It is designated chemically as (2S)-2-amino-3-[3-hydroxy-4-(phosphonooxy)phenyl] propanoic acid. Its empirical formula is C9H12NO7P, and its structural formula is:](https://fda.report/DailyMed/28e806e4-951c-40a9-9f0c-d0929caf054c/vyalev-02.jpg)
VYALEV (foscarbidopa and foslevodopa) injection is a sterile, preservative-free solution for subcutaneous infusion. VYALEV is supplied in a 10 mL single-dose glass vial that contains 120 mg foscarbidopa and 2,400 mg foslevodopa per 10 mL (12 mg foscarbidopa and 240 mg foslevodopa per mL). VYALEV may contain sodium hydroxide and/or hydrochloric acid to adjust the pH to approximately 7.4.
-
12
CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
VYALEV is a prodrug combination of foscarbidopa (carbidopa-4´-monophosphate) and foslevodopa (levodopa-4´-monophosphate). Foscarbidopa and foslevodopa are converted in vivo to carbidopa and levodopa.
Carbidopa
When levodopa is administered orally, it is rapidly decarboxylated to dopamine in extracerebral tissues so that only a small portion of a given dose is transported unchanged to the central nervous system. Carbidopa inhibits the decarboxylation of peripheral levodopa, making more levodopa available for delivery to the brain.
Levodopa
Levodopa is the metabolic precursor of dopamine, does cross the blood-brain barrier, and presumably is converted to dopamine in the brain. This is thought to be the mechanism whereby levodopa treats symptoms of Parkinson's disease.
12.2 Pharmacodynamics
Because the decarboxylase inhibiting activity is limited to extracerebral tissues, administration of carbidopa with levodopa makes more levodopa available to the brain. The addition of carbidopa to levodopa reduces the peripheral effects (e.g., nausea and vomiting) due to decarboxylation of levodopa; however, carbidopa does not decrease the adverse reactions due to the central effects of levodopa.
12.3 Pharmacokinetics
Absorption
VYALEV is administered directly into the subcutaneous space and is converted to carbidopa and levodopa by alkaline phosphatase. In a phase 1 study in healthy volunteers, carbidopa and levodopa were detectable in plasma within 30 minutes at the first pharmacokinetic (PK) collection point. In healthy volunteer subjects the steady state levodopa was achieved within 2 hours when VYALEV dosing was delivered as a loading dose followed by continuous infusion.
Healthy volunteers were administered VYALEV to different subcutaneous sites (i.e., abdomen, arm, and thigh) using a 3-way crossover design. PK analysis from this study showed that the 3 sites provided comparable carbidopa and levodopa exposure suggesting VYALEV absorption is similar at the different subcutaneous sites.
Effect of Food
VYALEV bypasses the gut, so consumption of food does not change absorption or systemic exposure of carbidopa/levodopa.
Distribution
Both foslevodopa and foscarbidopa have binding to plasma proteins between 24% to 26%.
Levodopa
Levodopa is distributed between erythrocytes and plasma in approximately 1:1 ratio. Levodopa binding to plasma proteins is< 10%. Levodopa is transported into the brain by the carrier mechanism for large neutral amino acids.
Carbidopa
Carbidopa is approximately 36% bound to plasma protein. Carbidopa does not cross the blood-brain barrier.
Metabolism and Excretion
The foscarbidopa and foslevodopa from VYALEV are converted by alkaline phosphatases into carbidopa and levodopa.
Levodopa
Levodopa is mainly eliminated via metabolism by the aromatic amino acid decarboxylase (AAAD) and the catechol-O-methyl-transferase (COMT) enzymes. Other routes of metabolism are transamination and oxidation. The decarboxylation of levodopa to dopamine by AAAD is the major enzymatic pathway when no enzyme inhibitor is co-administered. O-methylation of levodopa by COMT forms 3-O-methyldopa. When administered with carbidopa, the elimination half-life of levodopa is approximately 1.5 hours.
Carbidopa
Carbidopa is metabolized to two main metabolites (α-methyl-3-methoxy-4-hydroxyphenylpropionic acid and α-methyl-3,4-dihydroxyphenylpropionic acid). These 2 metabolites are primarily eliminated in the urine unchanged or as glucuronide conjugates. Unchanged carbidopa accounts for 30% of the total urinary excretion. The elimination half-life of carbidopa is approximately 2 hours.
-
13
NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In rat, oral administration of carbidopa and levodopa for two years resulted in no evidence of carcinogenicity. VYALEV contains hydrazine, a degradation product of carbidopa. In published studies, hydrazine has been demonstrated to be carcinogenic in multiple animal species. Increases in liver (adenoma, carcinoma) and lung (adenoma, adenocarcinoma) tumors have been reported with oral administration of hydrazine in mouse, rat, and hamster.
Mutagenesis
Carbidopa was positive in the in vitro Ames test, in the presence and absence of metabolic activation, and the in vitro mouse lymphoma tk assay in the absence of metabolic activation but was negative in the in vivo mouse micronucleus assay.
In published studies, hydrazine was reported to be positive in in vitro genotoxicity (Ames, chromosomal aberration in mammalian cells, and mouse lymphoma tk) assays and in the in vivo mouse micronucleus assay.
Impairment of Fertility
In reproduction studies, no effects on fertility were observed in rats receiving carbidopa and levodopa.
-
14
CLINICAL STUDIES
The efficacy of VYALEV was established in a 12-week, randomized, double-blind, double-dummy, active-controlled, multicenter study (Study 1; NCT04380142) in patients with advanced Parkinson’s disease (PD). Study 1 enrolled patients who were responsive to levodopa treatment, had motor fluctuations inadequately controlled by their current medications, and who experienced a minimum of 2.5 hours of “Off” time per day as assessed by PD diaries. A total of 141 patients were randomized in 1:1 ratio and received either 24-hour/day continuous subcutaneous administration of VYALEV plus oral placebo capsules (N=74) or 24-hour/day continuous subcutaneous administration of placebo solution plus oral encapsulated carbidopa-levodopa immediate-release (IR) tablets (N=67).
Patients had a mean age of 66.4 years and a mean disease duration of 8.6 years. Most (93%) of the patients were white, 2% were Asian, 3% Black and 70% of the patients were male. At baseline, approximately 74% of patients in the VYALEV group and 66% of patients in the oral IR carbidopa-levodopa group were taking at least 1 or more classes of PD medications other than carbidopa-levodopa.
The primary clinical outcome measure was the mean change from baseline to Week 12 in the total daily mean “On” time without troublesome dyskinesia (defined as "On" time without dyskinesia plus "On" time with non-troublesome dyskinesia) based on PD diary.
The key secondary clinical outcome measure was the mean change from baseline to Week 12 in the total daily mean “Off” time. The “On” and “Off” time were normalized to a daily 16-hour awake period. Daily normalized "Off" and "On" times are averaged over valid PD diary days for each visit to obtain the average daily normalized times. VYALEV demonstrated statistically significant improvements from baseline to Week 12 in "On" time without troublesome dyskinesia compared with the oral IR carbidopa-levodopa group (p=0.0083; Table 3). VYALEV also demonstrated statistically significant improvements from baseline to Week 12 in “Off” time compared with the oral IR carbidopa-levodopa group (p=0.0054; Table 3).
Table 3. Change from Baseline to Week 12 in Primary and Key Secondary Measures Oral IR carbidopa-levodopab
(N=67)VYALEV
(N=73)Primary Measure “On” time without troublesome dyskinesia (hours)a Baseline Mean
(SD)9.49
(2.62)9.20
(2.42)Change from Baseline to Endpoint Week 12 Mean
(SD)0.85
(3.46)3.36
(3.62)LS Mean (SE) of Change 0.97
(0.50)2.72
(0.52)LS Mean (SE) of Difference 1.75 (0.65) P value 0.0083 Secondary Measure “Off” time (hours)a Baseline Mean
(SD)5.91
(1.88)6.34
(2.27)Change from Baseline to Endpoint Week 12 Mean
(SD)-0.93
(3.31)-3.41
(3.76)LS Mean (SE) of Change -0.96
(0.49)-2.75
(0.50)LS Mean (SE) of Difference -1.79 (0.63) P value 0.0054 LS = least squares; SD = standard deviation; SE = standard error.
a Derived from Parkinson’s Disease (PD) diary.
b Oral immediate release carbidopa-levodopa tablets.Figure 1 shows results over time according to treatment for the efficacy variable (mean change from baseline to week 12 in the total daily mean normalized “On” time without troublesome dyskinesia based on PD diary).
Figure 1. LS Mean Change (±SE) from Baseline in “On” Time Without Troublesome Dyskinesia over 12 Weeks
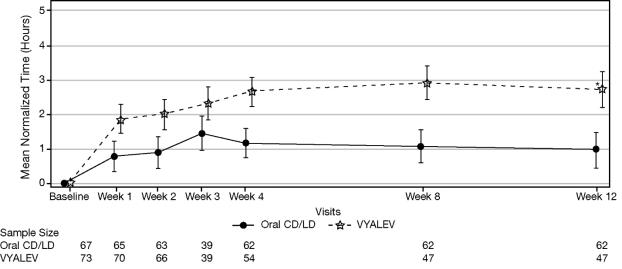
* p ≤ 0.01. P value reflects comparison between treatment groups
CD = carbidopa; LD = levodopa; LS = least squares; SE = standard error
Note - Week 3 was an optional visit.
Figure 2 shows results over time according to treatment for the efficacy variable (mean change from baseline to week 12 in the total daily mean normalized “Off” time based on PD diary).
Figure 2. LS Mean Change (±SE) from Baseline in “Off” Time over 12 Weeks
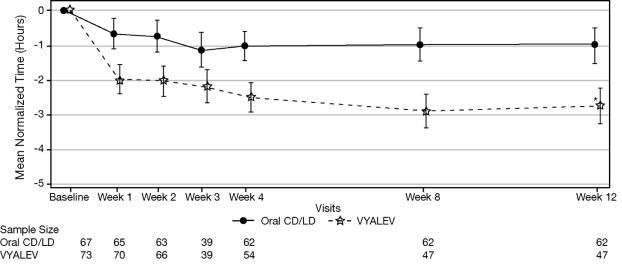
* p ≤ 0.01. P value reflects comparison between treatment groups
CD = carbidopa; LD = levodopa; LS = least squares; SE = standard error
Note - Week 3 was an optional visit.
-
16
HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
VYALEV injection contains 120 mg foscarbidopa and 2,400 mg foslevodopa per 10 mL (12 mg foscarbidopa and 240 mg foslevodopa per mL) and is a colorless to yellow to brown (may have a purple or red tint), and clear to slightly opalescent solution. Each single-dose glass vial is filled with approximately 10 mL of solution and is fitted with a grey rubber stopper, aluminum crimp cap, and turquoise plastic flip-off cap.
The rubber stopper on the vial does not contain natural rubber latex.
Carton of 7 VYALEV vials: NDC: 0074-0501-01
The VYAFUSER pump used to administer VYALEV (foscarbidopa and foslevodopa) is provided separately.
16.2 Storage and Handling
- Keep the medication vials in the outer carton to protect the vials from breaking.
- Store VYALEV refrigerated at 2°C to 8°C (36°F to 46°F).
- VYALEV may be stored at room temperature up to a maximum of 30°C (86°F) for a single period of up to 28 days.
- Once VYALEV has been stored at room temperature, do not return the product to the refrigerator.
- If stored at room temperature, discard VYALEV if not used within 28 days.
- Once VYALEV has been stored at room temperature, do not return the product to the refrigerator.
- Do not freeze.
- Do not shake.
- Keep the medication vials in the outer carton to protect the vials from breaking.
-
17
PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Administration Information
Refer patients to the Instructions for Use for complete administration instructions. Inform patients of aseptic technique and of subcutaneous administration site selection and rotation [see Dosage and Administration (2.3)]. Instruct the patient to record the date when VYALEV is first removed from the refrigerator in the space provided on the carton [see Storage and Handling (16.2)].
Interruption of VYALEV Infusion
Inform patients that if they disconnect the pump for less than 1 hour (e.g., to shower or for a short medical procedure), a new infusion set (tubing and cannula) or rotation of the infusion site is not needed before resuming infusion, unless medically indicated. Instruct the patient to stop the pump and disconnect the tubing. The syringe can remain attached to the pump until the tubing is reconnected. Refer the patient to the Patient Instructions for Use for additional information.
Inform patients that if they have a prolonged interruption of therapy lasting more than 1 hour, a new infusion set (tubing and cannula) should be used, and rotation to a different infusion site is required before resuming infusion. In addition, if VYALEV is interrupted for more than 3 hours, advise patients to administer a loading dose with either VYALEV or oral immediate-release carbidopa and levodopa [see Dosage and Administration (2.2)]. Instruct patients to have oral carbidopa and levodopa available in case treatment with VYALEV is interrupted for 1 hour or longer.
Falling Asleep during Activities of Daily Living and Somnolence
Alert patients to the potential sedating effects caused by VYALEV, including somnolence and the possibility of falling asleep while engaged in activities of daily living. Because somnolence is a common adverse reaction with potentially serious consequences, patients should not drive a car, operate machinery, or engage in other potentially dangerous activities until they have gained sufficient experience with VYALEV to gauge whether it affects their mental and/or motor performance adversely. Advise patients that if increased somnolence or episodes of falling asleep during activities of daily living (e.g., conversations, eating, driving a motor vehicle, etc.) are experienced at any time during treatment, they should not drive or participate in potentially dangerous activities until they have contacted their healthcare professional.
Advise patients of possible additive effects when patients are taking other sedating medications, alcohol, or other central nervous system depressants (e.g., benzodiazepines, antipsychotics, antidepressants, etc.) in combination with VYALEV or when taking a concomitant medication that increases plasma levels of levodopa [see Warnings and Precautions (5.1)].
Hallucinations/Psychosis/Confusion
Inform patients that they may experience hallucinations (unreal visions, sounds, or sensations) and other symptoms of psychosis while taking VYALEV. Tell patients to report hallucinations, abnormal thinking, psychotic behavior, or confusion to their healthcare professional promptly should they develop [see Warnings and Precautions (5.2)].
Impulse Control/Compulsive Behaviors
Advise patients that they may experience impulse control and/or compulsive behaviors while taking VYALEV. Advise patients to inform their healthcare professional if they develop new or increased gambling urges, sexual urges, uncontrolled spending, binge or compulsive eating, or other urges while being treated with VYALEV [see Warnings and Precautions (5.3)].
Infusion Site Reactions and Infections
Advise patients to contact their healthcare professional if they notice signs of inflammation or infection at the infusion site, such as local spreading of redness, swelling, warmth, pain, discoloration when they apply pressure to the area, and/or fever. Tell patients to follow aseptic techniques while using VYALEV and to regularly change the infusion site (at least every third day), using a new infusion set. Advise patients to make sure the new infusion site is at least 1 inch (2.5 cm) from a site used in the last 12 days. Instruct patients to remove the cannula if an infection at the infusion site occurs and to contact their healthcare provider. Inform patients that they may need to change the infusion site more often than every third day if they notice any of the above-mentioned signs of infection [see Warnings and Precautions (5.4)].
Withdrawal-Emergent Hyperpyrexia and Confusion
Advise patients to contact their healthcare professional before stopping VYALEV. Tell patients to inform their healthcare professional if they develop withdrawal symptoms such as fever, confusion, or severe muscle stiffness [see Warnings and Precautions (5.5)].
Dyskinesia
Inform patients that VYALEV may cause or exacerbate pre-existing dyskinesias [see Warnings and Precautions (5.6)].
Pregnancy
Advise patients to notify their healthcare provider if they become pregnant during treatment or plan to become pregnant during treatment [see Use in Specific Populations (8.1)].
Lactation
Advise patients to notify their healthcare provider if they are breastfeeding or plan to breastfeed [see Use in Specific Populations (8.2)].
Manufactured for:
AbbVie Inc.
North Chicago, IL 60064 U.S.A
VYALEV and its design are trademarks of AbbVie AB.
VYAFUSER™ is a trademark of AbbVie AB.
©2024 AbbVie. All rights reserved.
20065514 10-2024 -
MEDICATION GUIDE
Medication Guide
VYALEVTM (vye-uh-lev)
(foscarbidopa and foslevodopa)
injection, for subcutaneous useWhat is the most important information I should know about VYALEV?
VYALEV may cause serious side effects, including:
- Falling asleep during activities of daily living and somnolence
- Hallucinations/psychosis, or confusion
- Impulse control, compulsive behaviors
- Infusion site reactions and infections
- Cardiovascular ischemic events
See “What are the possible side effects of VYALEV?” for more information about side effects.What is VYALEV?
VYALEV is a prescription medicine used for treatment of advanced Parkinson’s disease in adults. VYALEV contains two medicines, foscarbidopa and foslevodopa.
It is not known if VYALEV is safe and effective in children.Do not use VYALEV if you:
- take a medicine called a nonselective monoamine oxidase (MAO) inhibitor or have taken a nonselective MAO inhibitor within the last 14 days.
Before using VYALEV, tell your healthcare provider about all your medical conditions, including if you:
- feel sleepy or have fallen asleep suddenly during the day.
- have or have been seeing things that are not there, hearing sounds or feeling sensations that are not real (hallucinations)
- have or have had unusual urges such as problems with gambling, compulsive eating, compulsive shopping, and increased sex drive.
- have trouble controlling your muscles (dyskinesia).
- have eye problems that cause increased pressure in your eye (glaucoma).
- drink alcohol. Alcohol can increase the chance that VYALEV will make you feel sleepy or fall asleep when you should be awake.
- have or have had heart problems, an abnormal heart rate or have had a heart attack in the past.
- have or have had high blood pressure (hypertension).
- are pregnant or plan to become pregnant. It is not known if VYALEV can harm your unborn baby.
- are breastfeeding or plan to breastfeed. Talk to your healthcare provider about the best way to feed your baby if you take VYALEV.
Using VYALEV with certain other medicines may affect each other and cause serious side effects.
Especially tell your healthcare provider if you take:
- medicines used to treat high blood pressure (hypertension)
- medicines used to treat depression called nonselective monoamine oxidase (MAO) inhibitor or have taken one within the last 14 days
- dopamine D2 receptor antagonists (antipsychotics or metoclopramide), and isoniazid
How should I use VYALEV? -
See the detailed “Instructions for Use of VYALEV” that comes with VYALEV for information on how to prepare syringes of VYALEV for use in the pump, and how to properly throw away (dispose of) used syringes.
-
See the detailed “Patient Instructions for Use of VYAFUSER Pump” that comes with your pump for complete instructions on how to use the delivery system.
- Use VYALEV exactly as your healthcare provider tells you to use it.
- Only use VYALEV by yourself after you have been shown the right way to use VYALEV. Ask your healthcare provider if you have any questions.
- Your prescribed dose of VYALEV will be programmed into your pump by a healthcare provider and should only be changed by your healthcare provider.
- Do not stop using VYALEV unless you are told to do so by your healthcare provider.
- Talk with your healthcare provider about what to do in case you are unable to use VYALEV infusion.
- Keep a supply of backup oral Parkinson’s disease medicines that contain levodopa and carbidopa with you at all times.
- VYALEV is given continuously over 24 hours under the skin by a pump.
- VYALEV can be stopped for brief periods of time, such as when taking a shower or short medical procedure. Make sure to change your infusion set (tubing and cannula) and rotate to a different infusion site if you stop the infusion for longer than 1 hour.
- If VYALEV has been stopped for less than 1 hour you will not need a new infusion set (tubing and cannula) and you will not need to rotate to a different infusion site.
- If your infusions have been stopped for longer than 3 hours, consider also giving yourself a loading dose to quickly get your symptoms back under control.
- Have oral carbidopa and levodopa available in case your treatment with VYALEV is stopped for 1 hour or longer.
- You may call 866-4-VYALEV (866-489-2538) for additional help or go to www.vyalev.com.
What should I avoid while using VYALEV? - Do not drive, operate machinery, or do other activities until you know how VYALEV affects you. Sleepiness and falling asleep suddenly caused by VYALEV can happen as late as 1 year after you start your treatment.
What are the possible side effects of VYALEV?
- See “What is the most important information I should know about VYALEV?”
-
Seeing things that are not there, hearing sounds or feeling sensations that are not real (hallucinations). This is a common and sometimes serious side effect. Hallucinations can happen in people who use VYALEV. Tell your healthcare provider if you have hallucinations.
-
Unusual urges. Some people taking certain medicines to treat Parkinson’s disease, including VYALEV, have reported problems, such as gambling, compulsive eating, compulsive shopping, and increased sex drive.
If you or your family members notice that you are having unusual urges or behaviors, talk to your healthcare provider.
- Infusion site reactions and infections. This is a common and sometimes serious side effect. Some people using VYALEV have had reactions and infections at the infusion site. Remove your cannula and call your healthcare provider if you have any of the following symptoms of an infection.
- local spreading of redness
- swelling
- change in color when pressing on the area
- pain
- warmth
- fever
- To prevent infection, clean your infusion site the way your healthcare provider has shown you (aseptic technique) and change your infusion site at least every 3 days using a new infusion site that is at least 1 inch from a site you used in the last 12 days.
- Withdrawal-emergent fever (hyperpyrexia) and confusion. Call your healthcare provider if you have any of the following symptoms after stopping VYALEV:
- fever
- confusion
- severe muscle stiffness
-
Uncontrolled sudden movements (dyskinesia). This is a common and sometimes serious side effect. If you have new dyskinesia, or your dyskinesia gets worse, tell your healthcare provider. This may be a sign that your dose of VYALEV or other medicines to control your Parkinson’s disease may need to be adjusted.
-
Heart attack or other heart problems. Tell your healthcare provider if you have had increased blood pressure, a fast or irregular heartbeat or chest pain.
- Worsening of the increased pressure in your eye (glaucoma). The pressure in your eyes should be checked after starting VYALEV.
Tell your healthcare provider if you have any side effect that bothers you or does not go away.
These are not all of the possible side effects of VYALEV. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store VYALEV?
- Keep the vials in the outer carton to protect the vials from breaking.
- Store VYALEV in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Record the date when VYALEV is first removed from the refrigerator in the space provided on the carton.
- VYALEV may be stored at room temperature up to a maximum of 86°F (30°C) for a single period of up to 28 days.
- After VYALEV has been stored at room temperature, do not return the medicine to the refrigerator.
- If stored at room temperature, throw away (dispose of) VYALEV if not used within 28 days.
- Do not freeze.
- Do not shake.
- If VYALEV is stored in the refrigerator, take one VYALEV vial out of the carton and out of the refrigerator 30 minutes before use. Use the VYALEV at room temperature or you may not get the right amount of the medicine.
General information about the safe and effective use of VYALEV
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use VYALEV for a condition for which it was not prescribed. Do not give VYALEV to other people, even if they have the same symptoms that you have. It may harm them.
If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about VYALEV that is written for healthcare professionals.What are the ingredients in VYALEV?
Active ingredients: foscarbidopa and foslevodopa
Inactive ingredients: Sterile water for injection. Sodium hydroxide and hydrochloric acid may be used to adjust the pH to 7.4.
Manufactured for AbbVie Inc. North Chicago, IL 60064 U.S.A.
©2024 AbbVie. All rights reserved.
VYALEV and its design are trademarks of AbbVie AB.
For more information, call 1-866-4-VYALEV (1-866-489-2538) or go to www.VYALEV.com.This Medication Guide has been approved by the U.S. Food and Drug Administration
2006551510/2024 - Falling asleep during activities of daily living and somnolence
-
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
VYALEV™ [vye-uh-lev]
(foscarbidopa and foslevodopa)
injection, for subcutaneous use
Read this Instructions for Use before you start using VYALEV and each time you get a new refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment. This Instructions for Use contains information on how to prepare VYALEV.
Before using VYALEV:
- Only use VYALEV by yourself after you have been shown the right way to use VYALEV and the delivery system (see the Patient Instructions for Use of VYAFUSER Pump).
- Call your healthcare provider or call (866) 4-VYALEV or (866) 489-2538 for help or go to www.VYALEV.com.
How should I store VYALEV?
- Keep the vials in the outer carton to protect the vials from breaking.
- Store VYALEV in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Record the date when VYALEV is first removed from the refrigerator in the space provided on the carton.
- VYALEV may be stored at room temperature up to a maximum of 86°F (30°C) for a single period of up to 28 days.
- After VYALEV has been stored at room temperature, do not return the medicine to the refrigerator.
- If stored at room temperature, throw away (dispose of) VYALEV if not used within 28-days.
- Do not freeze.
- Do not shake.
- If VYALEV is stored in the refrigerator, take one VYALEV vial out of the carton and out of the refrigerator 30 minutes before use. Use the VYALEV at room temperature or you may not get the right amount of the medicine.
Keep VYALEV and all medicines out of the reach of children.
Important Information:
Solution
-
The VYALEV solution color may have changes in color and does not affect how well the medicine works. It may be colorless, or may have different colors anywhere between light yellow and brown, possibly with purple or red tint. It also may become darker while in the syringe.
- If refrigerated before use, remove the solution vial from refrigerated storage and allow to sit at room temperature out of direct sunlight for 30 minutes.
-
Do not add any other liquid (dilute) the VYALEV solution or fill the syringe with anything other than what your healthcare provider prescribed.
- Do not use VYALEV if the expiration date (EXP:) shown on the carton and vial has passed.
Disposable Parts (Vial Adapter and Syringe)
- A new vial adapter must be used with each new vial of VYALEV.
- Do not use the VYALEV solution if it has been in the syringe for more than 24 hours.
A. Transfer VYALEV from Solution Vial to Syringe
1. Make sure your work space is clean.
Note: This will help to avoid contamination.
2. Gather supplies, including (see Figure A):
- Solution vial
- Syringe (not included in VYALEV carton)
- Vial adapter (not included in VYALEV carton)
- Alcohol pads (not included in VYALEV carton)
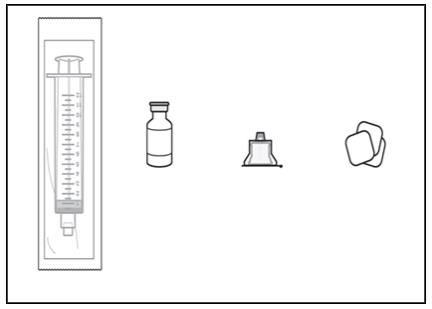
Figure A

3. Check over the parts for expiration and for any packaging damage. This should include:
- Solution vial (see Figure B)
- Vial adapter
- Syringe
- Check if the solution is the VYALEV solution prescribed by your healthcare provider.
-
Do not use the VYALEV solution, vial adapter, or syringe if it is expired.
- Do not use any parts if their sterile packaging has been damaged before use.
Note: The product packaging for the infusion set, vial adapter, and syringe show that they are sterile and how they were sterilized.
Figure B
4. Check over the contents of the VYALEV vial (see Figure C). Check the following:
- No cloudiness of the liquid.
- No particles seen in the liquid.
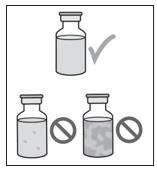
Figure C
- Do not use if the VYALEV solution is cloudy or contains flakes or particles.
Note: If refrigerated before use, remove the VYALEV solution vial from refrigerated storage and allow to sit at room temperature, out of direct sunlight, for 30 minutes.
- If refrigerated, do not warm VYALEV (in solution vial or syringe) in any way other than letting it warm at room temperature. For example, do not warm in a microwave or in hot water.
5. Wash your hands with soap and water and dry them (see Figure D).
Figure D
6. Prepare VYALEV solution vial.
a. Remove the vial cap (see Figure E).
Figure E
b. Wipe the top of the solution vial with an alcohol pad and allow to dry (see Figure F).
Note: This will help to avoid contamination.
Figure F
7. Attach the vial adapter to the solution vial.
Read your Vial Adapter Instructions for Use for detailed steps.

Vial Adapter
8. Prepare the syringe.
- To decrease the risk of infections, do not let the tip of any disposable parts come into contact with any unclean surfaces. If the tip of the vial adapter or syringe comes into contact with an unclean surface, throw (discard) it away and get a new one.
- Get a new syringe and remove it from its packaging.
- Push up on the rubber plunger to fully push out all air (see Figure G).
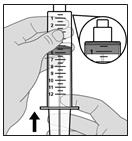
Figure G
9. While holding the vial adapter firmly, attach the syringe to the vial adapter by pushing and then screwing it into place (see Figure H).
- Do not overtighten.
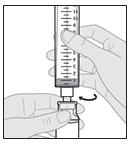
Figure H
10. Hold the syringe pointing straight up (vertically) with the solution vial above the syringe (see Figure I).
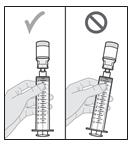
Figure I
11. Withdraw all of the medicine from the vial into the syringe.
- While holding the syringe firmly in one hand, pull down the plunger rod with the other hand to withdraw all of the medicine in the solution vial into the syringe to around the 12 mL mark, or until you see air at the tip of the syringe (see Figure J).
Note: It is important to hold the syringe pointing straight up.
Note: Always withdraw all of the medicine in the solution vial into the syringe.
Note: You will see air (head space) at the tip of the syringe.
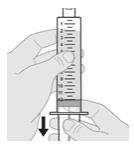
Figure J
12. Check for air bubbles.
- If there are large air bubbles, they must be removed. Air may affect if you get the right amount of medicine (your dose).
- As seen in Figure K, small bubbles are okay and the air at the tip of the syringe (the head space) is expected.
- As seen in Figure L, larger air bubbles are not okay. While the air at the top of the syringe (the head space) is expected, the larger bubbles are not.
-
If you see large air bubbles, continue with Section B: Manually Remove Air Bubbles.
- If you see small air bubbles or do not see any air bubbles, skip the next section and go to Section C: Remove (Purge) Air from Syringe.

Figure K
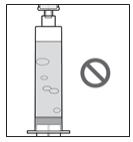
Figure L
B. Manually Remove Air Bubbles
13. Bring the bubbles together into a single air bubble.
a. Slowly and gently rotate the syringe and tilt it back and forth (see Figure M).
- Do not shake or tap the syringe to remove the air bubbles.
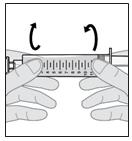
Figure M
Note: If there are still air bubbles, bring the bubbles together by gently rotating the syringe end over end (see Figure N).
b. When the large air bubbles are brought together into a single air bubble, continue with the next step.
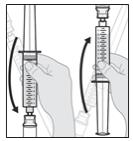
Figure N
C. Remove (Purge) Air from Syringe
14. Push air out of the syringe.
- With the solution vial still attached, point the syringe upward.
- Slowly push the air out of the syringe and into the vial (see Figure O).
- Continue pushing until all of the air is pushed out the syringe and into the solution vial and you can see that there is solution in the syringe tip.
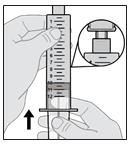
Figure O
Note: Some resistance will be felt as the air is pushed back into the solution vial.
Note: If the syringe is tilted slightly and not pointing straight up, you may see a small air bubble in the corner (see Figure P). This is okay.
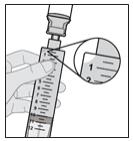
Figure P
15. Turn the syringe and solution vial upside down (invert) so that the solution vial is upright on the table (see Figure Q).
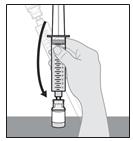
Figure Q
16. Disconnect the syringe from the vial adapter.
a. Hold the vial adapter firmly with one hand and the barrel of the syringe with the other.
b. Unscrew the syringe from the vial adapter (see Figure R).
Note: When disconnecting the syringe from the Vial, do not push the plunger or else the solution will leak.
c. Place the syringe on a clean surface, making sure the syringe tip does not contact an unclean surface.
- To decrease the risk of infections, do not let the tip of any disposable part come into contact with any unclean surfaces. If the tip of the vial adapter or syringe comes into contact with an unclean surface, throw it away (discard) and get a new one.
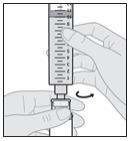
Figure R
17. Your syringe is now ready for use. Refer to your pump manual (Patient Instructions for Use of VYAFUSER Pump) for the next steps.

- Only use VYALEV by yourself after you have been shown the right way to use VYALEV and the delivery system (see the Patient Instructions for Use of VYAFUSER Pump).
-
INSTRUCTIONS FOR USE
Patient Instructions for Use
of VYAFUSER Pump
D. Disposing of VYALEV
18. Used solution vials with the vial adapters still attached should be thrown away (disposed of) in accordance with local regulations or as directed by your healthcare provider.
This Instructions for Use for VYALEV (foscarbidopa and foslevodopa) is to be used with the VYAFUSER pump (see the Patient Instructions for Use of VYAFUSER Pump).
Manufactured for:
AbbVie Inc. North Chicago, IL 60064 U.S.A.
VYALEV and its design are trademarks of AbbVie AB.
VYAFUSER™ is a trademark of AbbVie AB.
©2024 AbbVie. All rights reserved.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Approved: 10/2024
20065527 -
PRINCIPAL DISPLAY PANEL
NDC: 0074-0501-01
ATTENTION PHARMACIST:
Dispense the accompanying
Medication Guide to each patient.
VAYALEV™
foscarbidopa and
foslevodopa
Injection
120 mg and 2,400 mg per 10 mL
(12 mg and 240 mg per mL)
For subcutaneous use
7 x 10 mL single-dose vials
abbvie

-
INGREDIENTS AND APPEARANCE
VYALEV
foscarbidopa/foslevodopa injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0074-0501 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FOSCARBIDOPA (UNII: 5NT8HCA4OO) (FOSCARBIDOPA - UNII:5NT8HCA4OO) FOSCARBIDOPA 12 mg in 1 mL FOSLEVODOPA (UNII: 37NQZ0J76I) (FOSLEVODOPA - UNII:37NQZ0J76I) FOSLEVODOPA 240 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0074-0501-01 7 in 1 CARTON 04/12/2022 1 10 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216962 04/12/2022 Labeler - AbbVie Inc. (078458370)
Trademark Results [Vyalev]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VYALEV 98246135 not registered Live/Pending |
AbbVie AB 2023-10-30 |
 VYALEV 88640320 not registered Live/Pending |
AbbVie AB 2019-10-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.