0012 Aplicare Povidone-Iodine Solution (non-sterile)
Aplicare by
Drug Labeling and Warnings
Aplicare by is a Otc medication manufactured, distributed, or labeled by Aplicare Products, LLC, Medline Industries, LP. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
APLICARE- povidone-iodine solution
Aplicare Products, LLC
----------
0012 Aplicare Povidone-Iodine Solution (non-sterile)
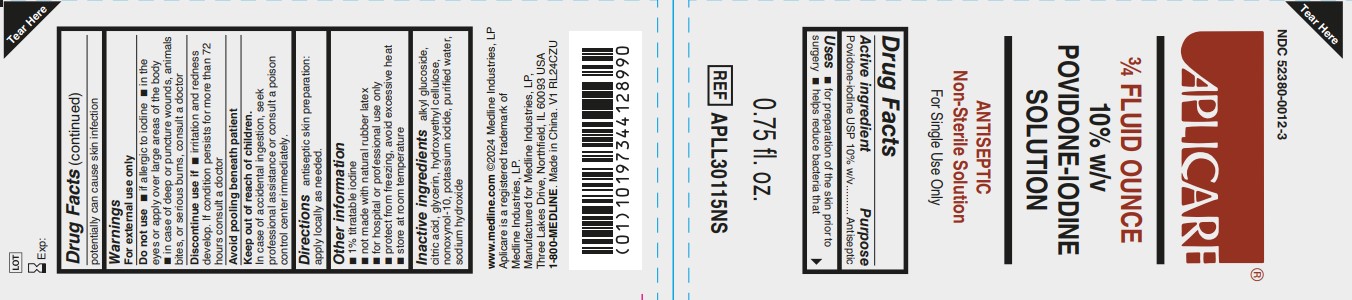
Uses
- for preparation of the skin prior to surgery
- helps reduce bacteria that potentially can cause skin infection
Warnings
For external use only
Do not use
- if allergic to iodine
- in the eyes or apply over large areas of the body
- in case of deep or puncture wounds, animals bites, or serious burns, consult a doctor
Other information
- 1% titratable iodine
- not made with natural rubber latex
- for hospital or professional use only
- protect from freezing, avoid excessive heat
- store at room temperature
Inactive ingredients
alkyl glucoside, citric acid, glycerin, hydroxyethyl cellulose, nonoxynol-10, potassium iodide, purified water, sodium hydroxide
| APLICARE
povidone-iodine solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Aplicare Products, LLC (081054904) |
| Registrant - Medline Industries, LP (025460908) |
Revised: 12/2025
Document Id: 44e780cc-13fe-dd55-e063-6294a90ad46a
Set id: 29049f69-1ffd-ca59-e063-6394a90a244f
Version: 3
Effective Time: 20251201
Trademark Results [Aplicare]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 APLICARE 87468700 5426290 Live/Registered |
MEDLINE INDUSTRIES, INC. 2017-05-30 |
 APLICARE 78632952 3085520 Live/Registered |
MEDLINE INDUSTRIES, INC. 2005-05-19 |
 APLICARE 78490074 3017931 Live/Registered |
MEDLINE INDUSTRIES, INC. 2004-09-27 |
 APLICARE 77039201 3397049 Live/Registered |
MEDLINE INDUSTRIES, INC. 2006-11-08 |
 APLICARE 74122266 1668208 Dead/Cancelled |
Aplicare, Inc. 1990-12-10 |
 APLICARE 73461083 1311965 Live/Registered |
Aplicare, Inc. 1984-01-16 |
 APLICARE 73461082 1311964 Live/Registered |
Aplicare, Inc. 1984-01-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.