CETIRIZINE HYDROCHLORIDE tablet, film coated
CETIRIZINE HYDROCHLORIDE by
Drug Labeling and Warnings
CETIRIZINE HYDROCHLORIDE by is a Otc medication manufactured, distributed, or labeled by Indoco Remedies Limited , INDOCO REMEDIES LIMITED. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT(S)
- PURPOSE
- USE(S)
-
WARNINGS
Do not use if you have ever had an allergic reaction to this product or any of its ingredients or to an antihistamine containing hydroxyzine.
Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
Ask a doctor or pharmacist before use if you are taking tranquilizers or sedatives.
When using this product
- drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
Stop use and ask a doctor if an allergic reaction to this product occurs. Seek medical help right away.
If pregnant or breast-feeding
- if breast-feeding: not recommended
- if pregnant: ask a health professional before use.
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS
adults and children 6 years and over
one 10 mg tablet once daily; do not take more than one 10 mg tablet in 24 hours. A 5 mg product may be appropriate for less severe symptoms.
adults 65 years and over
ask a doctor
children under 6 years of age
ask a doctor
consumers with liver or kidney disease
ask a doctor
Other information
- store between 20° to 25°C (68° to 77°F)
- do not use if foil inner seal is broken or missing
- FDA approved dissolution test specifications differ from USP
-
PRINCIPAL DISPLAY PANEL
Original Prescription Strength
NDC: 14445-152-30
Cetirizine Hydrochloride Tablets
ALLERGY
Cetirizine HCl tablets
10 mg /antihistamine
Indoor & Outdoor Allergies
24 hour Relief of
runny nose
sneezing
itchy, watery eyes
itchy throat or nose
30 Tablets
10 mg eachContainer Label:
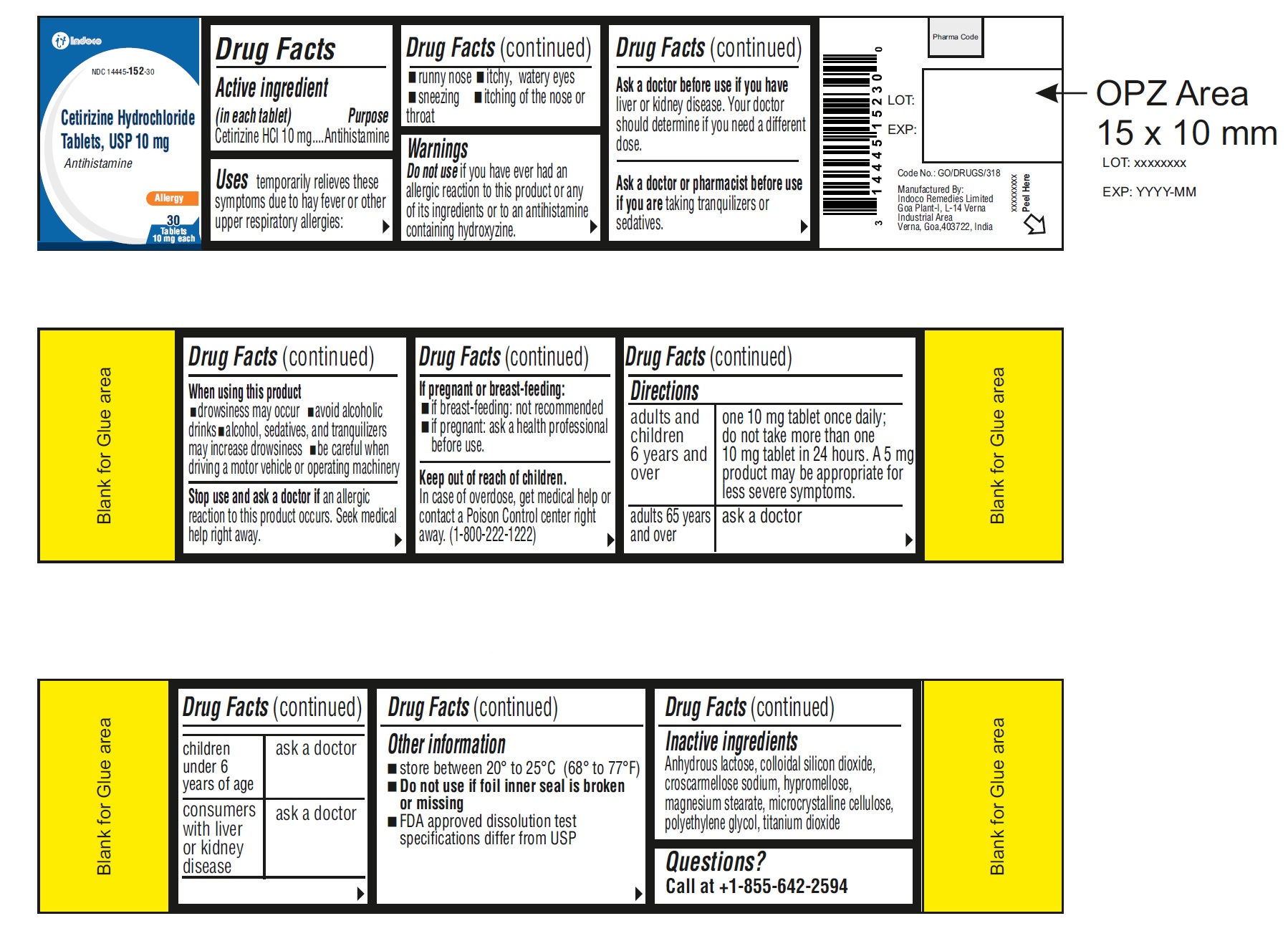
Carton Label:

-
INGREDIENTS AND APPEARANCE
CETIRIZINE HYDROCHLORIDE
cetirizine hydrochloride tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 14445-152 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETIRIZINE HYDROCHLORIDE (UNII: 64O047KTOA) (CETIRIZINE - UNII:YO7261ME24) CETIRIZINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) Product Characteristics Color WHITE Score 2 pieces Shape CAPSULE Size 9mm Flavor Imprint Code C10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 14445-152-30 1 in 1 CARTON 10/04/2024 1 30 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC: 14445-152-90 1 in 1 CARTON 10/04/2024 2 90 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC: 14445-152-01 1 in 1 CARTON 10/04/2024 3 100 in 1 BOTTLE; Type 0: Not a Combination Product 4 NDC: 14445-152-03 1 in 1 CARTON 10/04/2024 4 300 in 1 BOTTLE; Type 0: Not a Combination Product 5 NDC: 14445-152-05 1 in 1 CARTON 10/04/2024 5 500 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA218895 10/04/2024 Labeler - Indoco Remedies Limited (650445950) Establishment Name Address ID/FEI Business Operations INDOCO REMEDIES LIMITED 918608431 ANALYSIS(14445-152) , LABEL(14445-152) , MANUFACTURE(14445-152) , PACK(14445-152)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.