RINVOQ- upadacitinib tablet, extended release
Rinvoq by
Drug Labeling and Warnings
Rinvoq by is a Prescription medication manufactured, distributed, or labeled by AbbVie Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use RINVOQ safely and effectively. See full prescribing information for RINVOQ.
RINVOQ™ (upadacitinib) extended-release tablets, for oral use
Initial U.S. Approval: 2019WARNING: SERIOUS INFECTIONS, MALIGNANCY, AND THROMBOSIS
See full prescribing information for complete boxed warning.
- Serious infections leading to hospitalization or death, including tuberculosis and bacterial, invasive fungal, viral, and other opportunistic infections, have occurred in patients receiving RINVOQ. (5.1)
- If a serious infection develops, interrupt RINVOQ until the infection is controlled. (5.1)
- Prior to starting RINVOQ, perform a test for latent tuberculosis; if it is positive, start treatment for tuberculosis prior to starting RINVOQ. (5.1)
- Monitor all patients for active tuberculosis during treatment, even if the initial latent tuberculosis test is negative. (5.1)
- Lymphoma and other malignancies have been observed in patients treated with RINVOQ. (5.2)
- Thrombosis, including deep vein thrombosis, pulmonary embolism, and arterial thrombosis, have occurred in patients treated with Janus kinase inhibitors used to treat inflammatory conditions. (5.3)
INDICATIONS AND USAGE
RINVOQ is a Janus kinase (JAK) inhibitor indicated for the treatment of adults with moderately to severely active rheumatoid arthritis who have had an inadequate response or intolerance to methotrexate. (1)
Limitation of Use: Use of RINVOQ in combination with other JAK inhibitors, biologic DMARDs, or with potent immunosuppressants such as azathioprine and cyclosporine is not recommended. (1)
DOSAGE AND ADMINISTRATION
- The recommended dose of RINVOQ is 15 mg once daily. (2.1)
- RINVOQ may be used as monotherapy or in combination with methotrexate or other nonbiologic DMARDs. (2.1)
- Avoid initiation or interrupt RINVOQ if absolute lymphocyte count is less than 500 cells/mm3, absolute neutrophil count is less than 1000 cells/mm3, or hemoglobin level is less than 8 g/dL. (2.2, 2.3, 5.4)
DOSAGE FORMS AND STRENGTHS
Extended-release tablets: 15 mg (3)
CONTRAINDICATIONS
- None (4)
WARNINGS AND PRECAUTIONS
- Serious Infections: Avoid use of RINVOQ in patients with active, serious infection, including localized infections. (5.1)
- Malignancy: Consider the risks and benefits of RINVOQ treatment prior to initiating therapy in patients with a known malignancy. (5.2)
- Thrombosis: Consider the risks and benefits prior to treating patients who may be at increased risk of thrombosis. Promptly evaluate patients with symptoms of thrombosis and treat appropriately. (5.3)
- Gastrointestinal Perforations: Use with caution in patients who may be at increased risk. (5.4)
- Laboratory Monitoring: Recommended due to potential changes in lymphocytes, neutrophils, hemoglobin, liver enzymes and lipids. (5.5)
- Embryo-Fetal Toxicity: RINVOQ may cause fetal harm based on animal studies. Advise females of reproductive potential of the potential risk to a fetus and to use effective contraception. (5.6, 8.1, 8.3)
- Vaccinations: Avoid use of RINVOQ with live vaccines. (5.7)
ADVERSE REACTIONS
Adverse reactions (greater than or equal to 1%) are: upper respiratory tract infections, nausea, cough, and pyrexia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact AbbVie Inc. at 1-800-633-9110 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 8/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: SERIOUS INFECTIONS, MALIGNANCY, AND THROMBOSIS
1 INDICATIONS AND USAGE
1.1 Rheumatoid Arthritis
2 DOSAGE AND ADMINISTRATION
2.1 Dosage in Rheumatoid Arthritis
2.2 Important Administration Instructions
2.3 Dose Interruption
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Serious Infections
5.2 Malignancy
5.3 Thrombosis
5.4 Gastrointestinal Perforations
5.5 Laboratory Parameters
5.6 Embryo-Fetal Toxicity
5.7 Vaccination
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Strong CYP3A4 Inhibitors
7.2 Strong CYP3A4 Inducers
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: SERIOUS INFECTIONS, MALIGNANCY, AND THROMBOSIS
Patients treated with RINVOQ are at increased risk for developing serious infections that may lead to hospitalization or death [see Warnings and Precautions (5.1), Adverse Reactions (6.1)]. Most patients who developed these infections were taking concomitant immunosuppressants such as methotrexate or corticosteroids.
If a serious infection develops, interrupt RINVOQ until the infection is controlled.
- Active tuberculosis, which may present with pulmonary or extrapulmonary disease. Patients should be tested for latent tuberculosis before RINVOQ use and during therapy. Treatment for latent infection should be considered prior to RINVOQ use.
- Invasive fungal infections, including cryptococcosis and pneumocystosis.
- Bacterial, viral, including herpes zoster, and other infections due to opportunistic pathogens.
The risks and benefits of treatment with RINVOQ should be carefully considered prior to initiating therapy in patients with chronic or recurrent infection.
Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with RINVOQ, including the possible development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy [see Warnings and Precautions (5.1)].
Lymphoma and other malignancies have been observed in patients treated with RINVOQ [see Warnings and Precautions (5.2)].
Thrombosis, including deep venous thrombosis, pulmonary embolism, and arterial thrombosis have occurred in patients treated with Janus kinase inhibitors used to treat inflammatory conditions. Many of these adverse events were serious and some resulted in death. Consider the risks and benefits prior to treating patients who may be at increased risk. Patients with symptoms of thrombosis should be promptly evaluated and treated appropriately [see Warnings and Precautions (5.3)].
-
1 INDICATIONS AND USAGE
1.1 Rheumatoid Arthritis
RINVOQ™ (upadacitinib) is indicated for the treatment of adults with moderately to severely active rheumatoid arthritis who have had an inadequate response or intolerance to methotrexate.
Limitation of Use: Use of RINVOQ in combination with other JAK inhibitors, biologic DMARDs, or with potent immunosuppressants such as azathioprine and cyclosporine, is not recommended.
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosage in Rheumatoid Arthritis
The recommended oral dose of RINVOQ is 15 mg once daily with or without food [see Clinical Pharmacology (12.3)].
RINVOQ may be used as monotherapy or in combination with methotrexate or other nonbiologic DMARDs.
2.2 Important Administration Instructions
- RINVOQ initiation is not recommended in patients with an absolute lymphocyte count (ALC) less than 500 cells/mm3, absolute neutrophil count (ANC) less than 1000 cells/mm3, or hemoglobin level less than 8 g/dL [see Warnings and Precautions (5.4)].
- RINVOQ is not recommended for use in patients with severe hepatic impairment (Child-Pugh C) [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)].
- RINVOQ tablets should be swallowed whole. RINVOQ should not be split, crushed, or chewed.
2.3 Dose Interruption
RINVOQ treatment should be interrupted if a patient develops a serious infection until the infection is controlled [see Warnings and Precautions (5.1)].
Interruption of dosing may be needed for management of laboratory abnormalities as described in Table 1.
Table 1: Recommended Dose Interruptions for Laboratory Abnormalities Laboratory measure Action Absolute Neutrophil Count (ANC) Treatment should be interrupted if ANC is less than 1000 cells/mm3 and may be restarted once ANC return above this value Absolute Lymphocyte Count (ALC) Treatment should be interrupted if ALC is less than 500 cells/mm3 and may be restarted once ALC return above this value Hemoglobin (Hb) Treatment should be interrupted if Hb is less than 8 g/dL and may be restarted once Hb return above this value Hepatic transaminases Treatment should be interrupted if drug-induced liver injury is suspected - 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Serious Infections
Serious and sometimes fatal infections have been reported in patients receiving RINVOQ. The most frequent serious infections reported with RINVOQ included pneumonia and cellulitis [see Adverse Reactions (6.1)]. Among opportunistic infections, tuberculosis, multidermatomal herpes zoster, oral/esophageal candidiasis, and cryptococcosis, were reported with RINVOQ.
Avoid use of RINVOQ in patients with an active, serious infection, including localized infections. Consider the risks and benefits of treatment prior to initiating RINVOQ in patients:
- with chronic or recurrent infection
- who have been exposed to tuberculosis
- with a history of a serious or an opportunistic infection
- who have resided or traveled in areas of endemic tuberculosis or endemic mycoses; or
- with underlying conditions that may predispose them to infection.
Closely monitor patients for the development of signs and symptoms of infection during and after treatment with RINVOQ. Interrupt RINVOQ if a patient develops a serious or opportunistic infection. A patient who develops a new infection during treatment with RINVOQ should undergo prompt and complete diagnostic testing appropriate for an immunocompromised patient; appropriate antimicrobial therapy should be initiated, the patient should be closely monitored, and RINVOQ should be interrupted if the patient is not responding to antimicrobial therapy. RINVOQ may be resumed once the infection is controlled.
Patients should be screened for tuberculosis (TB) before starting RINVOQ therapy. RINVOQ should not be given to patients with active TB. Anti-TB therapy should be considered prior to initiation of RINVOQ in patients with previously untreated latent TB or active TB in whom an adequate course of treatment cannot be confirmed, and for patients with a negative test for latent TB but who have risk factors for TB infection.
Consultation with a physician with expertise in the treatment of TB is recommended to aid in the decision about whether initiating anti-TB therapy is appropriate for an individual patient.
Monitor patients for the development of signs and symptoms of TB, including patients who tested negative for latent TB infection prior to initiating therapy.
Viral reactivation, including cases of herpes virus reactivation (e.g., herpes zoster) and hepatitis B virus reactivation, were reported in clinical studies with RINVOQ [see Adverse Reactions (6.1)]. If a patient develops herpes zoster, consider temporarily interrupting RINVOQ until the episode resolves.
Screening for viral hepatitis and monitoring for reactivation should be performed in accordance with clinical guidelines before starting and during therapy with RINVOQ. Patients who were positive for hepatitis C antibody and hepatitis C virus RNA, were excluded from clinical studies. Patients who were positive for hepatitis B surface antigen or hepatitis B virus DNA were excluded from clinical studies. However, cases of hepatitis B reactivation were still reported in patients enrolled in the Phase 3 studies of RINVOQ. If hepatitis B virus DNA is detected while receiving RINVOQ, a liver specialist should be consulted.
5.2 Malignancy
Malignancies were observed in clinical studies of RINVOQ [see Adverse Reactions (6.1)]. Consider the risks and benefits of RINVOQ treatment prior to initiating therapy in patients with a known malignancy other than a successfully treated non-melanoma skin cancer (NMSC) or when considering continuing RINVOQ in patients who develop a malignancy.
NMSCs have been reported in patients treated with RINVOQ. Periodic skin examination is recommended for patients who are at increased risk for skin cancer.
5.3 Thrombosis
Thrombosis, including deep venous thrombosis, pulmonary embolism, and arterial thrombosis, have occurred in patients treated for inflammatory conditions with Janus kinase (JAK) inhibitors, including RINVOQ. Many of these adverse events were serious and some resulted in death.
Consider the risks and benefits of RINVOQ treatment prior to treating patients who may be at increased risk of thrombosis. If symptoms of thrombosis occur, patients should be evaluated promptly and treated appropriately.
5.4 Gastrointestinal Perforations
Events of gastrointestinal perforation have been reported in clinical studies with RINVOQ, although the role of JAK inhibition in these events is not known. In these studies, many patients with rheumatoid arthritis were receiving background therapy with Nonsteroidal Anti-Inflammatory Drugs (NSAIDs).
RINVOQ should be used with caution in patients who may be at increased risk for gastrointestinal perforation (e.g., patients with a history of diverticulitis or taking NSAIDs). Patients presenting with new onset abdominal symptoms should be evaluated promptly for early identification of gastrointestinal perforation.
5.5 Laboratory Parameters
Treatment with RINVOQ was associated with an increased incidence of neutropenia (ANC less than 1000 cells/mm3).
Evaluate neutrophil counts at baseline and thereafter according to routine patient management. Avoid initiation of or interrupt RINVOQ treatment in patients with a low neutrophil count (i.e., ANC less than 1000 cells/mm3) [see Dosage and Administration (2.2, 2.3)].
ALC less than 500 cells/mm3 were reported in RINVOQ clinical studies.
Evaluate lymphocyte counts at baseline and thereafter according to routine patient management. Avoid initiation of or interrupt RINVOQ treatment in patients with a low lymphocyte count (i.e., less than 500 cells/mm3) [see Dosage and Administration (2.2, 2.3)].
Decreases in hemoglobin levels to less than 8 g/dL were reported in RINVOQ clinical studies.
Evaluate hemoglobin at baseline and thereafter according to routine patient management. Avoid initiation of or interrupt RINVOQ treatment in patients with a low hemoglobin level (i.e., less than 8 g/dL) [see Dosage and Administration (2.2, 2.3)].
Treatment with RINVOQ was associated with increases in lipid parameters, including total cholesterol, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol [see Adverse Reactions (6.1)]. Elevations in LDL cholesterol decreased to pre-treatment levels in response to statin therapy. The effect of these lipid parameter elevations on cardiovascular morbidity and mortality has not been determined.
Patients should be monitored 12 weeks after initiation of treatment, and thereafter according to the clinical guidelines for hyperlipidemia. Manage patients according to clinical guidelines for the management of hyperlipidemia.
Treatment with RINVOQ was associated with increased incidence of liver enzyme elevation compared to placebo.
Evaluate at baseline and thereafter according to routine patient management. Prompt investigation of the cause of liver enzyme elevation is recommended to identify potential cases of drug-induced liver injury.
If increases in ALT or AST are observed during routine patient management and drug-induced liver injury is suspected, RINVOQ should be interrupted until this diagnosis is excluded.
5.6 Embryo-Fetal Toxicity
Based on findings in animal studies, RINVOQ may cause fetal harm when administered to a pregnant woman. Administration of upadacitinib to rats and rabbits during organogenesis caused increases in fetal malformations. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with RINVOQ and for 4 weeks following completion of therapy [see Use in Specific Populations (8.1, 8.3)].
5.7 Vaccination
Use of live, attenuated vaccines during, or immediately prior to, RINVOQ therapy is not recommended. Prior to initiating RINVOQ, it is recommended that patients be brought up to date with all immunizations, including prophylactic zoster vaccinations, in agreement with current immunization guidelines.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Serious Infections [see Warnings and Precautions (5.1)]
- Malignancy [see Warnings and Precautions (5.2)]
- Thrombosis [see Warnings and Precautions (5.3)]
- Gastrointestinal Perforations [see Warnings and Precautions (5.4)]
- Laboratory Parameters [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
A total of 3833 patients with rheumatoid arthritis were treated with upadacitinib in the Phase 3 clinical studies of whom 2806 were exposed for at least one year.
Patients could advance or switch to RINVOQ 15 mg from placebo, or be rescued to RINVOQ from active comparator or placebo from as early as Week 12 depending on the study design.
A total of 2630 patients received at least 1 dose of RINVOQ 15 mg, of whom 1860 were exposed for at least one year. In studies RA-I, RA-II, RA-III and RA-V, 1213 patients received at least 1 dose of RINVOQ 15 mg, of which 986 patients were exposed for at least one year, and 1203 patients received at least 1 dose of upadacitinib 30 mg, of which 946 were exposed for at least one year.
Table 2: Adverse Reactions Reported in greater than or equal to 1% of Rheumatoid Arthritis Patients Treated with RINVOQ 15 mg in Placebo-controlled Studies Adverse Reaction Placebo RINVOQ
15 mgn=1042
(%)n=1035
(%)Upper respiratory tract infection (URTI)* 9.5 13.5 Nausea 2.2 3.5 Cough 1.0 2.2 Pyrexia 0 1.2 *URTI includes: acute sinusitis, laryngitis, nasopharyngitis, oropharyngeal pain, pharyngitis, pharyngotonsillitis, rhinitis, sinusitis, tonsillitis, viral upper respiratory tract infection Other adverse reactions reported in less than 1% of patients in the RINVOQ 15 mg group and at a higher rate than in the placebo group through Week 12 included pneumonia, herpes zoster, herpes simplex (includes oral herpes), and oral candidiasis.
Four integrated datasets are presented in the Specific Adverse Reaction section:
Placebo-controlled Studies: Studies RA-III, RA-IV, and RA-V were integrated to represent safety through 12/14 weeks for placebo (n=1042) and RINVOQ 15 mg (n=1035). Studies RA-III and RA-V were integrated to represent safety through 12 weeks for placebo (n=390), RINVOQ 15 mg (n=385), upadacitinib 30 mg (n=384). Study RA-IV did not include the 30 mg dose and, therefore, safety data for upadacitinib 30 mg can only be compared with placebo and RINVOQ 15 mg rates from pooling studies RA-III and RA-V.
MTX-controlled Studies: Studies RA-I and RA-II were integrated to represent safety through 12/14 weeks for MTX (n=530), RINVOQ 15 mg (n=534), and upadacitinib 30 mg (n=529).
12-Month Exposure Dataset: Studies RA-I, II, III, and V were integrated to represent the long-term safety of RINVOQ 15 mg (n=1213) and upadacitinib 30 mg (n=1203).
Exposure adjusted incidence rates were adjusted by study for all the adverse events reported in this section.
Placebo-controlled Studies: In RA-III, RA-IV, and RA-V, infections were reported in 218 patients (95.7 per 100 patient-years) treated with placebo and 284 patients (127.8 per 100 patient-years) treated with RINVOQ 15 mg. In RA-III and RA-V, infections were reported in 99 patients (136.5 per 100 patient-years) treated with placebo, 118 patients (164.5 per 100 patient-years) treated with RINVOQ 15 mg, and 126 patients (180.3 per 100 patient-years) treated with upadacitinib 30 mg.
MTX-controlled Studies: Infections were reported in 127 patients (119.5 per 100 patient-years) treated with MTX monotherapy, 104 patients (91.8 per 100 patient-years) treated with RINVOQ 15 mg monotherapy, and 128 patients (115.1 per 100 patient-years) treated with upadacitinib 30 mg monotherapy.
12-Month Exposure Dataset: Infections were reported in 615 patients (83.8 per 100 patient-years) treated with RINVOQ 15 mg and 674 patients (99.7 per 100 patient-years) treated with upadacitinib 30 mg.
Placebo-controlled Studies: In RA-III, RA-IV, and RA-V, serious infections were reported in 6 patients (2.3 per 100 patient-years) treated with placebo, and 12 patients (4.6 per 100 patient-years) treated with RINVOQ 15 mg. In RA-III and RA-V, serious infections were reported in 1 patient (1.2 per 100 patient-years) treated with placebo, 2 patients (2.3 per 100 patient-years) treated with RINVOQ 15 mg, and 7 patients (8.2 per 100 patient-years) treated with upadacitinib 30 mg.
MTX-controlled Studies: Serious infections were reported in 2 patients (1.6 per 100 patient-years) treated with MTX monotherapy, 3 patients (2.4 per 100 patient-years) treated with RINVOQ 15 mg monotherapy, and 8 patients (6.4 per 100 patient-years) treated with upadacitinib 30 mg monotherapy.
12-Month Exposure Dataset: Serious infections were reported in 38 patients (3.5 per 100 patient-years) treated with RINVOQ 15 mg and 59 patients (5.6 per 100 patient-years) treated with upadacitinib 30 mg.
The most frequently reported serious infections were pneumonia and cellulitis.
Placebo-controlled Studies and MTX-controlled Studies: In the placebo-controlled period, there were no active cases of tuberculosis reported in the placebo, RINVOQ 15 mg, and upadacitinib 30 mg groups. In the MTX-controlled period, there were no active cases of tuberculosis reported in the MTX monotherapy, RINVOQ 15 mg monotherapy, and upadacitinib 30 mg monotherapy groups.
12-Month Exposure Dataset: Active tuberculosis was reported for 2 patients treated with RINVOQ 15 mg and 1 patient treated with upadacitinib 30 mg. Cases of extra-pulmonary tuberculosis were reported.
Opportunistic Infections (excluding tuberculosis)
Placebo-controlled Studies: In RA-III, RA-IV, and RA-V, opportunistic infections were reported in 3 patients (1.2 per 100 patient-years) treated with placebo, and 5 patients (1.9 per 100 patient-years) treated with RINVOQ 15 mg. In RA-III and RA-V, opportunistic infections were reported in 1 patient (1.2 per 100 patient-years) treated with placebo, 2 patients (2.3 per 100 patient-years) treated with RINVOQ 15 mg, and 6 patients (7.1 per 100 patient-years) treated with upadacitinib 30 mg.
MTX-controlled Studies: Opportunistic infections were reported in 1 patient (0.8 per 100 patient-years) treated with MTX monotherapy, 0 patients treated with RINVOQ 15 mg monotherapy, and 4 patients (3.2 per 100 patient-years) treated with upadacitinib 30 mg monotherapy.
12-Month Exposure Dataset: Opportunistic infections were reported in 7 patients (0.6 per 100 patient-years) treated with RINVOQ 15 mg and 15 patients (1.4 per 100 patient-years) treated with upadacitinib 30 mg.
Placebo-controlled Studies: In RA-III, RA-IV, and RA-V, malignancies excluding NMSC were reported in 1 patient (0.4 per 100 patient-years) treated with placebo, and 1 patient (0.4 per 100 patient-years) treated with RINVOQ 15 mg. In RA-III and RA-V, malignancies excluding NMSC were reported in 0 patients treated with placebo, 1 patient (1.1 per 100 patient-years) treated with RINVOQ 15 mg, and 3 patients (3.5 per 100 patient-years) treated with upadacitinib 30 mg.
MTX-controlled Studies: Malignancies excluding NMSC were reported in 1 patient (0.8 per 100 patient-years) treated with MTX monotherapy, 3 patients (2.4 per 100 patient-years) treated with RINVOQ 15 mg monotherapy, and 0 patients treated with upadacitinib 30 mg monotherapy.
12-Month Exposure Dataset: Malignancies excluding NMSC were reported in 13 patients (1.2 per 100 patient-years) treated with RINVOQ 15 mg and 14 patients (1.3 per 100 patient-years) treated with upadacitinib 30 mg.
Placebo-controlled Studies: There were no gastrointestinal perforations (based on medical review) reported in patients treated with placebo, RINVOQ 15 mg, and upadacitinib 30 mg.
MTX-controlled Studies: There were no cases of gastrointestinal perforations reported in the MTX and RINVOQ 15 mg group through 12/14 weeks. Two cases of gastrointestinal perforations were observed in the upadacitinib 30 mg group.
12-Month Exposure Dataset: Gastrointestinal perforations were reported in 1 patient treated with RINVOQ 15 mg and 4 patients treated with upadacitinib 30 mg.
Placebo-controlled Studies: In RA-IV, venous thrombosis (pulmonary embolism or deep vein thrombosis) was observed in 1 patient treated with placebo and 1 patient treated with RINVOQ 15 mg. In RA-V, venous thrombosis was observed in 1 patient treated with RINVOQ 15 mg. There were no observed cases of venous thrombosis reported in RA-III. No cases of arterial thrombosis were observed through 12/14 weeks.
MTX-controlled Studies: In RA-II, venous thrombosis was observed in 0 patients treated with MTX monotherapy, 1 patient treated with RINVOQ 15 mg monotherapy and 0 patients treated with upadacitinib 30 mg monotherapy through Week 14. In RA-II, no cases of arterial thrombosis were observed through 12/14 weeks. In RA-I, venous thrombosis was observed in 1 patient treated with MTX, 0 patients treated with RINVOQ 15 mg and 1 patient treated with upadacitinib 30 mg through Week 24. In RA-I, arterial thrombosis was observed in 1 patient treated with upadacitinib 30 mg through Week 24.
12-Month Exposure Dataset: Venous thrombosis events were reported in 5 patients (0.5 per 100 patient-years) treated with RINVOQ 15 mg and 4 patients (0.4 per 100 patient-years) treated with upadacitinib 30 mg. Arterial thrombosis events were reported in 0 patients treated with RINVOQ 15 mg and 2 patients (0.2 per 100 patient-years) treated with upadacitinib 30 mg.
Hepatic Transaminase Elevations
In placebo-controlled studies (RA-III, RA-IV, and RA-V) with background DMARDs, for up to 12/14 weeks, alanine transaminase (ALT) and aspartate transaminase (AST) elevations ≥ 3 x upper limit of normal (ULN) in at least one measurement were observed in 2.1% and 1.5% of patients treated with RINVOQ 15 mg, and in 1.5% and 0.7% of patients treated with placebo, respectively. In RA-III and RA-V, ALT and AST elevations ≥ 3 x ULN in at least one measurement were observed in 0.8% and 1.0% of patients treated with RINVOQ 15 mg, 1.0% and 0% of patients treated with upadacitinib 30 mg and in 1.3% and 1.0% of patients treated with placebo, respectively.
In MTX-controlled studies, for up to 12/14 weeks, ALT and AST elevations ≥ 3 x ULN in at least one measurement were observed in 0.8% and 0.4% of patients treated with RINVOQ 15 mg, 1.7% and 1.3% of patients treated with upadacitinib 30 mg and in 1.9% and 0.9% of patients treated with MTX, respectively.
Upadacitinib treatment was associated with dose-related increases in total cholesterol, triglycerides and LDL cholesterol. Upadacitinib was also associated with increases in HDL cholesterol. Elevations in LDL and HDL cholesterol peaked by Week 8 and remained stable thereafter. In controlled studies, for up to 12/14 weeks, changes from baseline in lipid parameters in patients treated with RINVOQ 15 mg and upadacitinib 30 mg, respectively, are summarized below:
- Mean LDL cholesterol increased by 14.81 mg/dL and 17.17 mg/dL.
- Mean HDL cholesterol increased by 8.16 mg/dL and 9.01 mg/dL.
- The mean LDL/HDL ratio remained stable.
- Mean triglycerides increased by 13.55 mg/dL and 14.44 mg/dL.
Creatine Phosphokinase Elevations
In placebo-controlled studies (RA-III, RA-IV, and RA-V) with background DMARDs, for up to 12/14 weeks, dose-related increases in creatine phosphokinase (CPK) values were observed. CPK elevations > 5 x ULN were reported in 1.0%, and 0.3% of patients over 12/14 weeks in the RINVOQ 15 mg and placebo groups, respectively. Most elevations >5 x ULN were transient and did not require treatment discontinuation. In RA-III and RA-V, CPK elevations > 5 x ULN were observed in 0.3% of patients treated with placebo, 1.6% of patients treated with RINVOQ 15 mg, and none in patients treated with upadacitinib 30 mg.
In placebo-controlled studies (RA-III, RA-IV, and RA-V) with background DMARDs, for up to 12/14 weeks, dose-related decreases in neutrophil counts, below 1000 cells/mm3 in at least one measurement occurred in 1.1% and <0.1% of patients in the RINVOQ 15 mg and placebo groups, respectively. In RA-III and RA-V, decreases in neutrophil counts below 1000 cells/mm3 in at least one measurement occurred in 0.3% of patients treated with placebo, 1.3% of patients treated with RINVOQ 15 mg, and 2.4% of patients treated with upadacitinib 30 mg. In clinical studies, treatment was interrupted in response to ANC less than 500 cells/mm3.
In placebo-controlled studies (RA-III, RA-IV, and RA-V) with background DMARDs, for up to 12/14 weeks, dose-related decreases in lymphocyte counts below 500 cells/mm3 in at least one measurement occurred in 0.9% and 0.7% of patients in the RINVOQ 15 mg and placebo groups, respectively. In RA-III and RA-V, decreases in lymphocyte counts below 500 cells/mm3 in at least one measurement occurred in 0.5% of patients treated with placebo, 0.5% of patients treated with RINVOQ 15 mg, and 2.4% of patients treated with upadacitinib 30 mg.
In placebo-controlled studies (RA-III, RA-IV, and RA-V) with background DMARDs, for up to 12/14 weeks, hemoglobin decreases below 8 g/dL in at least one measurement occurred in <0.1% of patients in both the RINVOQ 15 mg and placebo groups. In RA-III and RA-V, hemoglobin decreases below 8 g/dL in at least one measurement were observed in 0.3% of patients treated with placebo, and none in patients treated with RINVOQ 15 mg and upadacitinib 30 mg.
-
7 DRUG INTERACTIONS
7.1 Strong CYP3A4 Inhibitors
Upadacitinib exposure is increased when co-administered with strong CYP3A4 inhibitors (such as ketoconazole) [see Clinical Pharmacology (12.3)]. RINVOQ should be used with caution in patients receiving chronic treatment with strong CYP3A4 inhibitors.
7.2 Strong CYP3A4 Inducers
Upadacitinib exposure is decreased when co-administered with strong CYP3A4 inducers (such as rifampin), which may lead to reduced therapeutic effect of RINVOQ [see Clinical Pharmacology (12.3)]. Coadministration of RINVOQ with strong CYP3A4 inducers is not recommended.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
The limited human data on use of RINVOQ in pregnant women are not sufficient to evaluate a drug-associated risk for major birth defects or miscarriage. Based on animal studies, upadacitinib has the potential to adversely affect a developing fetus.
In animal embryo-fetal development studies, oral upadacitinib administration to pregnant rats and rabbits at exposures equal to or greater than approximately 1.6 and 15 times the maximum recommended human dose (MRHD), respectively, resulted in dose-related increases in skeletal malformations (rats only), an increased incidence of cardiovascular malformations (rabbits only), increased post-implantation loss (rabbits only), and decreased fetal body weights in both rats and rabbits. No developmental toxicity was observed in pregnant rats and rabbits treated with oral upadacitinib during organogenesis at approximately 0.3 and 2 times the exposure at the MRHD. In a pre- and post-natal development study in pregnant female rats, oral upadacitinib administration at exposures approximately 3 times the MRHD resulted in no maternal or developmental toxicity [see Animal Data].
The estimated background risks of major birth defects and miscarriage for the indicated population(s) are unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriages are 2-4% and 15-20%, respectively.
Disease-Associated Maternal and/or Embryo/Fetal Risk
Published data suggest that increased disease activity is associated with the risk of developing adverse pregnancy outcomes in women with rheumatoid arthritis. Adverse pregnancy outcomes include preterm delivery (before 37 weeks of gestation), low birth weight (less than 2500 g) infants, and small for gestational age at birth.
In an oral embryo-fetal development study, pregnant rats received upadacitinib at doses of 5, 25, and 75 mg/kg/day during the period of organogenesis from gestation day 6 to 17. Upadacitinib was teratogenic (skeletal malformations that consisted of misshapen humerus and bent scapula) at exposures equal to or greater than approximately 1.7 times the MRHD (on an AUC basis at maternal oral doses of 5 mg/kg/day and higher). Additional skeletal malformations (bent forelimbs/hindlimbs and rib/vertebral defects) and decreased fetal body weights were observed in the absence of maternal toxicity at an exposure approximately 84 times the MRHD (on an AUC basis at a maternal oral dose of 75 mg/kg/day).
In a second oral embryo-fetal development study, pregnant rats received upadacitinib at doses of 1.5 and 4 mg/kg/day during the period of organogenesis from gestation day 6 to 17. Upadacitinib was teratogenic (skeletal malformations that included bent humerus and scapula) at exposures approximately 1.6 times the MRHD (on an AUC basis at maternal oral doses of 4 mg/kg/day). No developmental toxicity was observed in rats at an exposure approximately 0.3 times the MRHD (on an AUC basis at a maternal oral dose of 1.5 mg/kg/day).
In an oral embryo-fetal developmental study, pregnant rabbits received upadacitinib at doses of 2.5, 10, and 25 mg/kg/day during the period of organogenesis from gestation day 7 to 19. Embryolethality, decreased fetal body weights, and cardiovascular malformations were observed in the presence of maternal toxicity at an exposure approximately 15 times the MRHD (on an AUC basis at a maternal oral dose of 25 mg/kg/day). Embryolethality consisted of increased post-implantation loss that was due to elevated incidences of both total and early resorptions. No developmental toxicity was observed in rabbits at an exposure approximately 2 times the MRHD (on an AUC basis at a maternal oral dose of 10 mg/kg/day).
In an oral pre- and post-natal development study, pregnant female rats received upadacitinib at doses of 2.5, 5, and 10 mg/kg/day from gestation day 6 through lactation day 20. No maternal or developmental toxicity was observed in either mothers or offspring, respectively, at an exposure approximately 3 times the MRHD (on an AUC basis at a maternal oral dose of 10 mg/kg/day).
8.2 Lactation
There are no data on the presence of upadacitinib in human milk, the effects on the breastfed infant, or the effects on milk production. Available pharmacodynamic/toxicological data in animals have shown excretion of upadacitinib in milk. When a drug is present in animal milk, it is likely that the drug will be present in human milk. Because of the potential for serious adverse reactions in the breastfed infant, advise patients that breastfeeding is not recommended during treatment with upadacitinib, and for 6 days (approximately 10 half-lives) after the last dose.
A single oral dose of 10 mg/kg radiolabeled upadacitinib was administered to lactating female Sprague-Dawley rats on post-partum days 7-8. Drug exposure was approximately 30-fold greater in milk than in maternal plasma based on AUC0-t values. Approximately 97% of drug-related material in milk was parent drug.
8.3 Females and Males of Reproductive Potential
Verify the pregnancy status of females of reproductive potential prior to starting treatment with RINVOQ [see Use in Specific Populations (8.1)].
Based on animal studies, upadacitinib may cause embryo-fetal harm when administered to pregnant women [see Use in Specific Populations (8.1)]. Advise female patients of reproductive potential to use effective contraception during treatment with RINVOQ and for 4 weeks after the final dose.
8.4 Pediatric Use
The safety and efficacy of RINVOQ in children and adolescents aged 0 to 18 years have not yet been established. No data are available.
8.5 Geriatric Use
Of the 4381 patients treated in the five Phase 3 clinical studies, a total of 906 rheumatoid arthritis patients were 65 years of age or older, including 146 patients 75 years and older. No differences in effectiveness were observed between these patients and younger patients; however, there was a higher rate of overall adverse events in the elderly.
8.6 Renal Impairment
No dose adjustment is required in patients with mild, moderate or severe renal impairment. The use of RINVOQ has not been studied in subjects with end stage renal disease [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
No dose adjustment is required in patients with mild (Child Pugh A) or moderate (Child Pugh B) hepatic impairment. RINVOQ is not recommended for use in patients with severe hepatic impairment (Child Pugh C) [see Dosage and Administration (2.2) and Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
Upadacitinib was administered in clinical trials up to doses equivalent in daily AUC to 60 mg extended-release once daily. Adverse events were comparable to those seen at lower doses and no specific toxicities were identified. Approximately 90% of upadacitinib in the systemic circulation is eliminated within 24 hours of dosing (within the range of doses evaluated in clinical studies). In case of an overdose, it is recommended that the patient be monitored for signs and symptoms of adverse reactions. Patients who develop adverse reactions should receive appropriate treatment.
-
11 DESCRIPTION
RINVOQ is formulated with upadacitinib, a JAK inhibitor.
Upadacitinib has the following chemical name: (3S,4R)-3-Ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide hydrate (2:1).
The strength of upadacitinib is based on anhydrous upadacitinib. The solubility of upadacitinib in water is 38 to less than 0.2 mg/mL across a pH range of 2 to 9 at 37 oC.
Upadacitinib has a molecular weight of 389.38 g/mol and a molecular formula of C17H19F3N6O ½ H2O. The chemical structure of upadacitinib is:
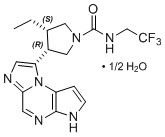
RINVOQ 15 mg extended-release tablets for oral administration are purple, biconvex oblong, with dimensions of 14 x 8 mm, and debossed with ‘a15’ on one side.
Each tablet contains the following inactive ingredients: microcrystalline cellulose, hypromellose, mannitol, tartaric acid, colloidal silicon dioxide, magnesium stearate, polyvinyl alcohol, polyethylene glycol, talc, titanium dioxide, ferrosoferric oxide, and iron oxide red.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Upadacitinib is a Janus kinase (JAK) inhibitor. JAKs are intracellular enzymes which transmit signals arising from cytokine or growth factor-receptor interactions on the cellular membrane to influence cellular processes of hematopoiesis and immune cell function. Within the signaling pathway, JAKs phosphorylate and activate Signal Transducers and Activators of Transcription (STATs) which modulate intracellular activity including gene expression. Upadacitinib modulates the signaling pathway at the point of JAKs, preventing the phosphorylation and activation of STATs.
JAK enzymes transmit cytokine signaling through their pairing (e.g., JAK1/JAK2, JAK1/JAK3, JAK1/TYK2, JAK2/JAK2, JAK2/TYK2). In a cell-free isolated enzyme assay, upadacitinib had greater inhibitory potency at JAK1 and JAK2 relative to JAK3 and TYK2. In human leukocyte cellular assays, upadacitinib inhibited cytokine-induced STAT phosphorylation mediated by JAK1 and JAK1/JAK3 more potently than JAK2/JAK2 mediated STAT phosphorylation. However, the relevance of inhibition of specific JAK enzymes to therapeutic effectiveness is not currently known.
12.2 Pharmacodynamics
Inhibition of IL-6 induced STAT3 and IL-7 induced STAT5 phosphorylation
In healthy volunteers, the administration of upadacitinib (immediate release formulation) resulted in a dose- and concentration-dependent inhibition of IL-6 (JAK1/JAK2)-induced STAT3 and IL-7 (JAK1/JAK3)-induced STAT5 phosphorylation in whole blood. The maximal inhibition was observed 1 hour after dosing which returned to near baseline by the end of dosing interval.
Treatment with upadacitinib was associated with a small, transient increase in mean ALC from baseline up to Week 36 which gradually returned to, at or near baseline levels with continued treatment.
In the controlled period, small decreases from baseline in mean IgG and IgM levels were observed with upadacitinib treatment; however, the mean values at baseline and at all visits were within the normal reference range.
At 6 times the mean maximum exposure of the 15 mg once daily dose, there was no clinically relevant effect on the QTc interval.
12.3 Pharmacokinetics
Upadacitinib plasma exposures are proportional to dose over the therapeutic dose range. Steady-state plasma concentrations are achieved within 4 days with minimal accumulation after multiple once-daily administrations.
Following oral administration of upadacitinib extended-release formulation, upadacitinib is absorbed with a median Tmax of 2 to 4 hours.
Coadministration of upadacitinib with a high-fat/ high-calorie meal had no clinically relevant effect on upadacitinib exposures (increased AUCinf by 29% and Cmax by 39%). In clinical trials, upadacitinib was administered without regard to meals [see Dosage and Administration (2.1)].
Upadacitinib is 52% bound to plasma proteins. Upadacitinib partitions similarly between plasma and blood cellular components with a blood to plasma ratio of 1.0.
Upadacitinib metabolism is mediated by mainly CYP3A4 with a potential minor contribution from CYP2D6. The pharmacologic activity of upadacitinib is attributed to the parent molecule. In a human radiolabeled study, unchanged upadacitinib accounted for 79% of the total radioactivity in plasma while the main metabolite detected (product of monooxidation followed by glucuronidation) accounted for 13% of the total plasma radioactivity. No active metabolites have been identified for upadacitinib.
Following single dose administration of [14C]-upadacitinib immediate-release solution, upadacitinib was eliminated predominantly as the unchanged parent substance in urine (24%) and feces (38%). Approximately 34% of upadacitinib dose was excreted as metabolites. Upadacitinib mean terminal elimination half-life ranged from 8 to 14 hours.
Body Weight, Gender, Race, and Age
Body weight, gender, race, ethnicity, and age did not have a clinically meaningful effect on upadacitinib exposure [see Use in Specific Populations (8.5)].
Renal impairment has no clinically relevant effect on upadacitinib exposure. Upadacitinib AUCinf was 18%, 33%, and 44% higher in subjects with mild, moderate, and severe renal impairment, respectively, compared to subjects with normal renal function. Upadacitinib Cmax was similar in subjects with normal and impaired renal function.
Mild (Child-Pugh A) and moderate (Child-Pugh B) hepatic impairment has no clinically relevant effect on upadacitinib exposure. Upadacitinib AUCinf was 28% and 24% higher in subjects with mild and moderate hepatic impairment, respectively, compared to subjects with normal liver function. Upadacitinib Cmax was unchanged in subjects with mild hepatic impairment and 43% higher in subjects with moderate hepatic impairment compared to subjects with normal liver function. Upadacitinib was not studied in patients with severe hepatic impairment (Child-Pugh C).
Potential for Other Drugs to Influence the Pharmacokinetics of Upadacitinib
Upadacitinib is metabolized in vitro by CYP3A4 with a minor contribution from CYP2D6. The effect of co-administered drugs on upadacitinib plasma exposures is provided in Table 3 [see Drug Interactions (7)].
Table 3: Change in Pharmacokinetics of Upadacitinib in the Presence of Co-administered Drugs Co-
administered
DrugRegimen
of Co-
administered
DrugRatio
(90% CI)aCmax AUC Methotrexate 10 to 25 mg/week 0.97
(0.86-1.09)0.99
(0.93- 1.06)Strong CYP3A4 inhibitor:
Ketoconazole400 mg once
daily x 6 days1.70
(1.55-1.89)1.75
(1.62-1.88)Strong CYP3A4 inducer:
Rifampin600 mg once
daily x 9 days0.49
(0.44-0.55)0.39
(0.37-0.42)OATP1B inhibitor:
Rifampin600 mg single dose 1.14
(1.02-1.28)1.07
(1.01-1.14)CI: Confidence interval
a Ratios for Cmax and AUC compare co-administration of the medication with upadacitinib vs. administration of upadacitinib alone.pH modifying medications (e.g., antacids or proton pump inhibitors) are not expected to affect upadacitinib plasma exposures based on in vitro assessments and population pharmacokinetic analyses. CYP2D6 metabolic phenotype had no effect on upadacitinib pharmacokinetics (based on population pharmacokinetic analyses), indicating that inhibitors of CYP2D6 have no clinically relevant effect on upadacitinib exposures.
Potential for Upadacitinib to Influence the Pharmacokinetics of Other Drugs
In vitro studies indicate that upadacitinib does not inhibit or induce the activity of cytochrome P450 (CYP) enzymes (CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A4) at clinically relevant concentrations. In vitro studies indicate that upadacitinib does not inhibit the transporters P-gp, BCRP, OATP1B1, OATP1B3, OCT1, OCT2, OAT1, OAT3, MATE1, and MATE2K at clinically relevant concentrations.
Clinical studies indicate that upadacitinib has no clinically relevant effects on the pharmacokinetics of co-administered drugs. Summary of results from clinical studies which evaluated the effect of upadacitinib on other drugs is provided in Table 4.
Table 4: Change in Pharmacokinetics of Co-administered Drugs or In Vivo Markers of CYP Activity in the Presence of Upadacitinib Co-administered
Drug or CYP
Activity MarkerMultiple-Dose
Regimen of
UpadacitinibRatio
(90% CI)aCmax AUC Methotrexate 6 mg to 24 mg BIDb 1.03
(0.86-1.23)1.14
(0.91-1.43)Sensitive CYP1A2 Substrate:
Caffeine30 mg QDc 1.13
(1.05-1.22)1.22
(1.15-1.29)Sensitive CYP3A Substrate:
Midazolam30 mg QDc 0.74
(0.68-0.80)0.74
(0.68-0.80)Sensitive CYP2D6 Substrate:
Dextromethorphan30 mg QDc 1.09
(0.98-1.21)1.07
(0.95-1.22)Sensitive CYP2C9 Substrate:
S-Warfarin30 mg QDc 1.07
(1.02-1.11)1.11
(1.07-1.15)Sensitive CYP2C19 Marker:
5-OH Omeprazole to
Omeprazole metabolic ratio30 mg QDc -- 1.09
(1.00-1.19)CYP2B6 Substrate:
Bupropion30 mg QDc 0.87
(0.79-0.96)0.92
(0.87-0.98)Rosuvastatin 30 mg QDc 0.77
(0.63-0.94)0.67
(0.56-0.82)Atorvastatin 30 mg QDc 0.88
(0.79-0.97)0.77
(0.70-0.85)Ethinylestradiol 30 mg QDc 0.96
(0.89-1.02)1.11
(1.04-1.19)Levonorgestrel 30 mg QDc 0.96
(0.87-1.06)0.96
(0.85-1.07)CYP: cytochrome P450; CI: Confidence interval; BID: twice daily; QD: once daily
a Ratios for Cmax and AUC compare co-administration of the medication with upadacitinib vs. administration of medication alone.
b Immediate-release formulation
c Extended-release formulation -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenic potential of upadacitinib was evaluated in Sprague-Dawley rats and Tg.rasH2 mice. No evidence of tumorigenicity was observed in male or female rats that received upadacitinib for up to 101 weeks at oral doses up to 15 or 20 mg/kg/day, respectively (approximately 4 and 10 times the MRHD on an AUC basis, respectively). No evidence of tumorigenicity was observed in male or female Tg.rasH2 mice that received upadacitinib for 26 weeks at oral doses up to 20 mg/kg/day.
Upadacitinib tested negatively in the following genotoxicity assays: the in vitro bacterial mutagenicity assay (Ames assay), in vitro chromosome aberration assay in human peripheral blood lymphocytes, and in vivo rat bone marrow micronucleus assay.
Upadacitinib had no effect on fertility in male or female rats at oral doses up to 50 mg/kg/day in males and 75 mg/kg/day in females (approximately 42 and 84 times the MRHD in males and females, respectively, on an AUC basis). However, maintenance of pregnancy was adversely affected at oral doses of 25 mg/kg/day and 75 mg/kg/day based upon dose-related findings of increased post-implantation losses (increased resorptions) and decreased numbers of mean viable embryos per litter (approximately 22 and 84 times the MRHD on an AUC basis, respectively). The number of viable embryos was unaffected in female rats that received upadacitinib at an oral dose of 5 mg/kg/day and were mated to males that received the same dose (approximately 2 times the MRHD on an AUC basis).
-
14 CLINICAL STUDIES
The efficacy and safety of RINVOQ 15 mg once daily were assessed in five Phase 3 randomized, double-blind, multicenter studies in patients with moderately to severely active rheumatoid arthritis and fulfilling the ACR/EULAR 2010 classification criteria. Patients over 18 years of age were eligible to participate. The presence of at least 6 tender and 6 swollen joints and evidence of systemic inflammation based on elevation of hsCRP was required at baseline. Although other doses have been studied, the recommended dose of RINVOQ is 15 mg once daily.
Study RA-I (NCT02706873) was a 24-week monotherapy trial in 947 patients with moderately to severely active rheumatoid arthritis who were naïve to methotrexate (MTX). Patients received RINVOQ 15 mg or upadacitinib 30 mg once daily or MTX as monotherapy. At Week 26, non-responding patients on upadacitinib could be rescued with the addition of MTX, while patients on MTX could be rescued with the addition of blinded RINVOQ 15 mg or upadacitinib 30 mg once daily. The primary endpoint was the proportion of patients who achieved an ACR50 response at Week 12. Key secondary endpoints included disease activity score (DAS28-CRP) ≤3.2 at Week 12, DAS28-CRP <2.6 at Week 24, change from baseline in HAQ-DI at Week 12, and change from baseline in van der Heijde-modified total Sharp Score (mTSS) at Week 24.
Study RA-II (NCT02706951) was a 14-week monotherapy trial in 648 patients with moderately to severely active rheumatoid arthritis who had an inadequate response to MTX. Patients received RINVOQ 15 mg or upadacitinib 30 mg once daily monotherapy or continued their stable dose of MTX monotherapy. At Week 14, patients who were randomized to MTX were advanced to RINVOQ 15 mg or upadacitinib 30 mg once daily monotherapy in a blinded manner based on pre-determined assignment at baseline. The primary endpoint was the proportion of patients who achieved an ACR20 response at Week 14. Key secondary endpoints included DAS28-CRP ≤3.2, DAS28-CRP <2.6, and change from baseline in HAQ-DI at Week 14.
Study RA-III (NCT02675426) was a 12-week trial in 661 patients with moderately to severely active rheumatoid arthritis who had an inadequate response to conventional disease modifying anti-rheumatic drugs (cDMARDs). Patients received RINVOQ 15 mg or upadacitinib 30 mg once daily or placebo added to background cDMARD therapy. At Week 12, patients who were randomized to placebo were advanced to RINVOQ 15 mg or upadacitinib 30 mg once daily in a blinded manner based on pre-determined assignment at baseline. The primary endpoint was the proportion of patients who achieved an ACR20 response at Week 12. Key secondary endpoints included DAS28-CRP ≤3.2, DAS28-CRP<2.6, and change from baseline in HAQ-DI at Week 12.
Study RA-IV (NCT02629159) was a 48-week trial in 1629 patients with moderately to severely active rheumatoid arthritis who had an inadequate response to MTX. Patients received RINVOQ 15 mg once daily, active comparator, or placebo added to background MTX. From Week 14, non-responding patients on RINVOQ 15 mg could be rescued to active comparator in a blinded manner, and non-responding patients on active comparator or placebo could be rescued to RINVOQ 15 mg in a blinded manner. At Week 26, all patients randomized to placebo were switched to RINVOQ 15 mg once daily in a blinded manner. The primary endpoint was the proportion of patients who achieved an ACR20 response at Week 12 versus placebo. Key secondary endpoints versus placebo included DAS28-CRP ≤3.2, DAS28-CRP <2.6, change from baseline in HAQ-DI at Week 12, and change from baseline in mTSS at Week 26.
Study RA-V (NCT02706847) was a 12-week trial in 499 patients with moderately to severely active rheumatoid arthritis who had an inadequate response or intolerance to biologic DMARDs. Patients received RINVOQ 15 mg or upadacitinib 30 mg once daily or placebo added to background cDMARD therapy. At Week 12, patients who were randomized to placebo were advanced to RINVOQ 15 mg or upadacitinib 30 mg once daily in a blinded manner based on pre-determined assignment at baseline. The primary endpoint was the proportion of patients who achieved an ACR20 response at Week 12. Key secondary endpoints included DAS28-CRP ≤3.2 and change from baseline in HAQ-DI at Week 12.
The percentages of RINVOQ-treated patients achieving ACR20, ACR50, and ACR70 responses, and DAS28(CRP) < 2.6 in all studies are shown in Table 5.
Patients treated with RINVOQ 15 mg, alone or in combination with cDMARDs, achieved higher ACR response rates compared to MTX monotherapy or placebo, respectively, at the primary efficacy timepoint (Table 5).
In Study IV, the percent of patients achieving ACR20 response by visit is shown in Figure 1.
In Studies RA-III and RA-V, higher ACR20 response rates were observed at 1 week with RINVOQ 15 mg versus placebo.
Treatment with RINVOQ 15 mg, alone or in combination with cDMARDs, resulted in greater improvements in the ACR components compared to MTX or placebo at the primary efficacy timepoint (Table 6).
Table 5: Clinical Response Study RA-I
MTX-NaïveStudy RA-II
MTX-IRStudy RA-III
cDMARD-IRStudy RA-IV
MTX-IRStudy RA-V
bDMARD-IRMonotherapy Monotherapy Background
cDMARDsBackground
MTXBackground
cDMARDsMTX RINVOQ
15 mg
%
Δ
(95% CI)MTX RINVOQ
15 mg
%
Δ
(95% CI)PBO RINVOQ
15 mg
%
Δ
(95% CI)PBO RINVOQ
15 mg
%
Δ
(95% CI)PBO RINVOQ
15 mg
%
Δ
(95% CI)N 314 317 216 217 221 221 651 651 169 164 Week ACR20 12a/14b 54 76
22 (14, 29)41 68
26 (17, 36)36 64
28 (19, 37)36 71
34 (29, 39)28 65
36 (26, 46)24c/26d 59 79
20 (13, 27)36 67
32 (27, 37)ACR50 12a/14b 28 52
24 (16, 31)15 42
27 (18, 35)15 38
23 (15, 31)15 45
30 (26, 35)12 34
22 (14, 31)24c/26d 33 60
27 (19, 34)21 54
33 (28, 38)ACR70 12a/14b 14 32
18 (12, 25)3 23
20 (14, 26)6 21
15 (9, 21)5 25
20 (16, 24)7 12
5 (-1, 11)24c/26d 18 44
26 (19, 33)10 35
25 (21, 29)DAS28-CRP <2.6 12a/14b 14 36
22 (15, 28)8 28
20 (13, 27)10 31
21 (14, 28)6 29
23 (19, 27)9 29
19 (11, 27)24c/26d 18 48
30 (23, 37)9 41
32 (27, 36)Abbreviations: ACR20 (or 50 or 70) = American College of Rheumatology ≥20% (or ≥50% or ≥70%) improvement; bDMARD = biologic disease-modifying anti-rheumatic drug; CRP = c-reactive protein; DAS28 = Disease Activity Score 28 joints; cDMARDs = conventional disease-modifying anti-rheumatic drugs; MTX = methotrexate; PBO = placebo; IR = inadequate responder
Patients who discontinued randomized treatment, or had cross-over between randomized treatments, or were missing data at week of evaluation were imputed as non-responders in the analyses.
a Study RA-I, Study RA-III, Study RA-IV, Study RA-V
b Study RA-II
c Study RA-I
d Study RA-IVTable 6: Components of ACR Response at Primary Efficacy Timepointa Study RA-I
MTX-NaïveStudy RA-IIb
MTX-IRStudy RA-III
cDMARD-IRStudy RA-IV
MTX-IRStudy RA-V
bDMARD-IRMonotherapy Monotherapy Background
cDMARDsBackground
MTXBackground
cDMARDsMTX RINVOQ
15 mgMTX RINVOQ
15 mgPBO RINVOQ
15 mgPBO RINVOQ
15 mgPBO RINVOQ
15 mgN 314 317 216 217 221 221 651 651 169 164 Number of tender joints (0-68) Baseline 26
(16)25
(14)25
(16)24
(15)25
(15)25
(14)26
(14)26
(15)28
(15)28
(16)Week
12/1413
(15)9
(12)15
(16)10
(13)16
(17)12
(14)16
(15)10
(13)18
(17)11
(14)Number of swollen joints (0-66) Baseline 17
(11)17
(10)17
(12)16
(11)15
(9)16
(10)16
(9)17
(10)16
(10)17
(11)Week
12/146
(8)5
(7)9
(11)6
(9)9
(10)7
(10)9
(9)5
(7)9
(10)6
(8)Painc Baseline 66
(21)68
(21)63
(21)62
(23)62
(21)64
(19)65
(21)66
(21)69
(21)68
(20)Week
12/1441
(25)31
(25)49
(25)36
(27)51
(26)33
(24)49
(25)33
(24)55
(28)41
(28)Patient global assessmentc Baseline 66
(21)67
(22)60
(22)62
(22)60
(20)63
(22)64
(21)64
(22)66
(23)67
(20)Week
12/1442
(25)31
(24)48
(26)37
(27)50
(26)32
(24)48
(24)33
(24)54
(28)40
(26)Disability Index (HAQ-DI)d Baseline 1.60
(0.67)1.60
(0.67)1.47
(0.66)1.47
(0.66)1.42
(0.63)1.48
(0.61)1.61
(0.61)1.63
(0.64)1.56
(0.60)1.67
(0.64)Week
12/141.08
(0.72)0.76
(0.69)1.19
(0.69)0.86
(0.67)1.13
(0.70)0.85
(0.66)1.28
(0.67)0.98
(0.68)1.33
(0.66)1.24
(0.77)Physician global assessmentc Baseline 69
(16)67
(17)62
(17)66
(18)64
(18)64
(16)66
(18)66
(17)67
(17)69
(17)Week
12/1432
(22)22
(19)37
(24)26
(21)41
(24)26
(21)41
(25)27
(21)39
(25)29
(22)CRP (mg/L) Baseline 21.2
(22.1)23.0
(27.4)14.5
(17.3)14.0
(16.5)12.6
(14.0)16.6
(19.2)18.0
(21.5)17.9
(22.5)16.3
(21.1)16.3
(18.6)Week
12/1410.9
(14.9)4.2
(8.8)12.8
(21.4)3.7
(7.8)13.1
(15.5)4.6
(9.6)16.2
(19.8)5.5
(10.9)13.9
(17.3)5.0
(14.0)Abbreviations: ACR = American College of Rheumatology; bDMARD = biologic disease-modifying anti-rheumatic drug; CRP = c-reactive protein; cDMARDs = conventional disease-modifying anti-rheumatic drugs; HAQ-DI = Health Assessment Questionnaire Disability Index; IR = inadequate responder; MTX = methotrexate; PBO = placebo
a Data shown are mean (standard deviation).
b Primary efficacy timepoint is at Week 14.
c Visual analog scale: 0 = best, 100 = worst.
d Health Assessment Questionnaire-Disability Index: 0=best, 3=worst; 20 questions; 8 categories: dressing and grooming, arising, eating, walking, hygiene, reach, grip, and activities.
Figure 1. Percent of Patients Achieving ACR20 in Study RA-IV
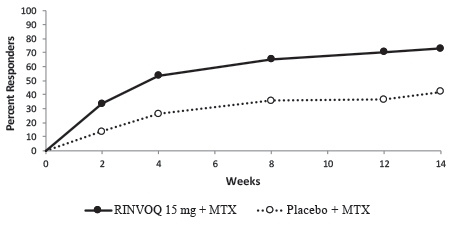
Abbreviations: ACR20 = American College of Rheumatology ≥20% improvement; MTX = methotrexate
Patients who discontinued randomized treatment, or were missing ACR20 results, or were lost-to-follow-up or withdrawn from the study were imputed as non-responders.In RA-I and RA-IV, a higher proportion of patients treated with RINVOQ 15 mg alone or in combination with cDMARDs, achieved DAS28-CRP < 2.6 compared to MTX or placebo at the primary efficacy timepoint (Table 7).
Table 7: Proportion of Patients with DAS28-CRP Less Than 2.6 with Number of Residual Active Joints at Primary Efficacy Timepoint Study RA-I
MTX-NaiveMonotherapy DAS28-CRP Less Than 2.6 MTX
N = 314RINVOQ 15 mg
N = 317Proportion of responders at Week 12 (n) 14% (43) 36% (113) Of responders, proportion with 0 active joints (n) 51% (22) 45% (51) Of responders, proportion with 1 active joint (n) 35% (15) 23% (26) Of responders, proportion with 2 active joints (n) 9% (4) 17% (19) Of responders, proportion with 3 or more active joints (n) 5% (2) 15% (17) Study RA-IV
MTX-IRBackground MTX DAS28-CRP Less Than 2.6 PBO
N = 651RINVOQ 15 mg
N = 651Proportion of responders at Week 12 (n) 6% (40) 29% (187) Of responders, proportion with 0 active joints (n) 60% (24) 48% (89) Of responders, proportion with 1 active joint (n) 20% (8) 23% (43) Of responders, proportion with 2 active joints (n) 15% (6) 13% (25) Of responders, proportion with 3 or more active joints (n) 5% (2) 16% (30) Abbreviations: CRP = c-reactive protein; DAS28 = Disease Activity Score 28 joints; MTX = methotrexate; PBO = placebo; IR = inadequate responder Inhibition of progression of structural joint damage was assessed using the modified Total Sharp Score (mTSS) and its components, the erosion score and joint space narrowing score, at Week 26 in Study RA-IV and Week 24 in Study RA-I. The proportion of patients with no radiographic progression (mTSS change from baseline ≤ 0) was also assessed.
In Study RA-IV, treatment with RINVOQ 15 mg inhibited the progression of structural joint damage compared to placebo in combination with cDMARDs at Week 26 (Table 8). Analyses of erosion and joint space narrowing scores were consistent with overall results.
In the placebo plus MTX group, 76% of the patients experienced no radiographic progression at Week 26 compared to 83% of the patients treated with RINVOQ 15 mg.
In Study RA-I, treatment with RINVOQ 15 mg monotherapy inhibited the progression of structural joint damage compared to MTX monotherapy at Week 24 (Table 8). Analyses of erosion and joint space narrowing scores were consistent with overall results.
In the MTX monotherapy group, 78% of the patients experienced no radiographic progression at Week 24 compared to 87% of the patients treated with RINVOQ 15 mg monotherapy.
Table 8: Radiographic Changes Study RA-IV
MTX-IRBackground MTX mTSS PBO
(N=651)
Mean (SD)RINVOQ 15 mg
(N=651)
Mean (SD)Estimated Difference vs PBO
at Week 26
(95% CI)1Baseline 35.9 (52) 34.0 (50) Week 262 0.78 (0.1) 0.15 (0.1) -0.63 (-0.92, -0.34) Study RA-I
MTX-naïveMonotherapy MTX
(N=309)
Mean (SD)RINVOQ 15 mg
(N=309)
Mean (SD)Estimated Difference vs MTX
at Week 24
(95% CI)3Baseline 13.3 (31) 18.1 (38) Week 244 0.67 (2.8) 0.14 (1.4) -0.53 (-0.85, -0.20) Abbreviations: mTSS = modified Total Sharp Score, MTX = methotrexate; PBO = placebo; SD = standard deviation; IR = inadequate responders; bDMARDs = biologic disease modifying anti-rheumatic drugs; LS = least squares; CI = confidence intervals
1 LS means and 95% CI based on a random coefficient model fit to the mTSS value adjusting for time, treatment group, prior bDMARDs use, treatment group-by-time interaction, with random slopes and random intercept.
2 Estimated linear rate of structural progression by Week 26 and standard errors are presented.
3 LS means and 95% CI based on a linear regression model fit to change from baseline in mTSS adjusting for treatment group, baseline mTSS, and geographic region.
4 Mean change from baseline and standard deviation are presented.Treatment with RINVOQ 15 mg, alone or in combination with cDMARDs, resulted in a greater improvement in physical function at Week 12/14 compared to all comparators as measured by HAQ-DI.
In all studies except for Study RA-V, patients receiving RINVOQ 15 mg had greater improvement from baseline in physical component summary (PCS) score, mental component summary (MCS) scores, and in all 8 domains of the Short Form Health Survey (SF-36) compared to placebo in combination with cDMARDs or MTX monotherapy at Week 12/14.
Fatigue was assessed by the Functional Assessment of Chronic Illness Therapy-Fatigue score (FACIT-F) in Studies RA-I, RA-III, and RA-IV. Improvement in fatigue at Week 12 was observed in patients treated with RINVOQ 15 mg compared to patients on placebo in combination with cDMARDs or MTX monotherapy.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
RINVOQ 15 mg extended-release tablets for oral administration are purple, biconvex oblong, with dimensions of 14 x 8 mm, and debossed with ‘a15’ on one side.
30 tablets in a bottle; NDC: 0074-2306-30
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Inform patients that they may be more likely to develop infections when taking RINVOQ. Instruct patients to contact their healthcare provider immediately during treatment if they develop any signs or symptoms of an infection [see Warnings and Precautions (5.1)].
Advise patients that the risk of herpes zoster is increased in patients taking RINVOQ and in some cases can be serious [see Warnings and Precautions (5.1)].
Inform patients that RINVOQ may increase their risk of certain cancers. Instruct patients to inform their healthcare provider if they have ever had any type of cancer [see Warnings and Precautions (5.2)].
Advise patients that events of DVT and PE have been reported in clinical studies with RINVOQ. Instruct patients to tell their healthcare provider if they develop any signs or symptoms of a DVT or PE [see Warnings and Precautions (5.3)].
Inform patients that RINVOQ may affect certain lab tests, and that blood tests are required before and during RINVOQ treatment [see Warnings and Precautions (5.5)].
Advise pregnant women and females of reproductive potential that exposure to RINVOQ during pregnancy may result in fetal harm. Advise females to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.6) and Use in Specific Populations (8.1)]. Advise females of reproductive potential that effective contraception should be used during treatment and for 4 weeks following the final dose of upadacitinib [see Use in Specific Populations (8.3)].
Advise women not to breastfeed during treatment with RINVOQ [see Use in Specific Populations (8.2)].
Advise patients not to chew, crush, or split RINVOQ tablets [see Dosage and Administration (2.2)].
Manufactured by: AbbVie Ireland NL B.V., Sligo, Ireland
Packed and Distributed by: AbbVie Inc., North Chicago, IL 60064
RINVOQ is a trademark of AbbVie Biotechnology Ltd.
©2019 AbbVie Inc.
03-B725 August 2019
-
MEDICATION GUIDE
-
PRINCIPAL DISPLAY PANEL
NDC: 0074-2306-30
Extended-Release Tablets 15 mg
Dispense in original packaging
NDC: 0074-2306-70
Extended-Release Tablets 15 mg
PROFESSIONAL SAMPLE NOT FOR SALE
Dispense in original packaging
-
INGREDIENTS AND APPEARANCE
RINVOQ
upadacitinib tablet, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0074-2306 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength upadacitinib (UNII: 4RA0KN46E0) (upadacitinib - UNII:4RA0KN46E0) upadacitinib 15 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MANNITOL (UNII: 3OWL53L36A) TARTARIC ACID (UNII: W4888I119H) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERROSOFERRIC OXIDE (UNII: XM0M87F357) FERRIC OXIDE RED (UNII: 1K09F3G675) Product Characteristics Color PURPLE Score no score Shape OVAL (biconvex oblong) Size 14mm Flavor Imprint Code a15 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0074-2306-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 08/16/2019 2 NDC: 0074-2306-70 14 in 1 BOTTLE; Type 0: Not a Combination Product 08/16/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA211675 08/16/2019 Labeler - AbbVie Inc. (078458370) Registrant - AbbVie Inc. (078458370)
Trademark Results [Rinvoq]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 RINVOQ 87320921 5908540 Live/Registered |
AbbVie Biotechnology Ltd. 2017-02-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.