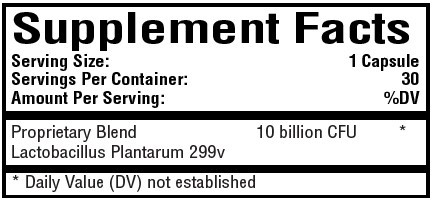
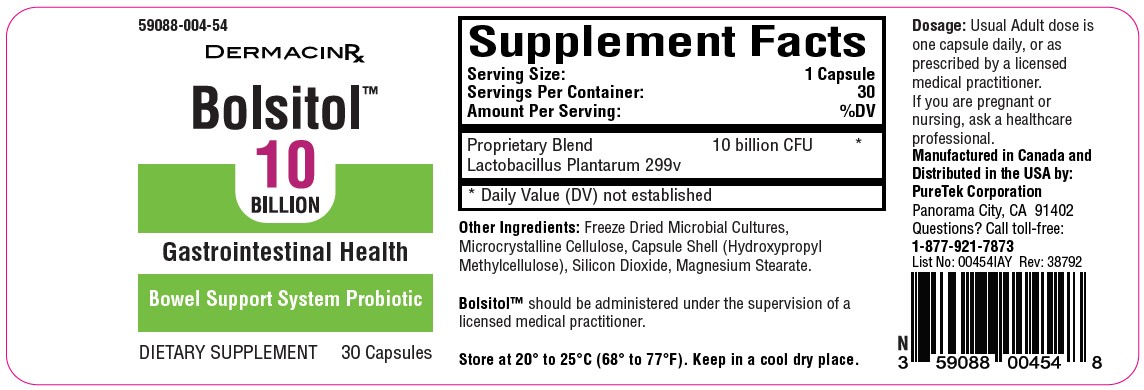
BOLSITOL- probiotic capsule
Bolsitol by
Drug Labeling and Warnings
Bolsitol by is a Other medication manufactured, distributed, or labeled by PureTek Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- STATEMENT OF IDENTITY
- DOSAGE
-
SAFE HANDLING
This product is contraindicated in patients with a known hypersensitivity to any of the ingredients.
Bolsitol™ should only be used under the direction and supervision of a licensed healthcare practitioner. Use with caution in patients that may have a medical condition, are pregnant, lactating, trying to conceive, under the age of 18, or taking medications.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Do not use if bottle seal is broken.
- STORAGE
-
HOW SUPPLIED HEALTH CLAIM
Bolsitol™, Probiotic
Dietary Supplement
NHRIC: 59088-004-54 *
Bottles contain 30 Capsules
Manufactured in Canada and
Distributed in the USA by:
PureTek Corporation
Panorama City, CA 91402
Questions? Call toll-free:
1-877-921-7873*Does not represent these product codes to be National Drug Codes (NDC). Product codes are formatted according to standard industry practice, to meet the formatting requirement by pedigree reporting and supply-chain control including pharmacies.
† The most appropriate way to ensure pedigree reporting consistent with these regulatory guidelines and safety monitoring is to dispense this product by prescription. This is not an Orange Book product. This product may be administered only under a physician’s supervision and all prescriptions using this product shall be pursuant to state statutes as applicable. The ingredients, indication or claims of this product are not to be construed to be drug claims.
- Bolsitol™
-
INGREDIENTS AND APPEARANCE
BOLSITOL
probiotic capsuleProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:59088-004 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LACTIPLANTIBACILLUS PLANTARUM 299V (UNII: PH2Q4E64WM) (LACTIPLANTIBACILLUS PLANTARUM 299V - UNII:PH2Q4E64WM) LACTIPLANTIBACILLUS PLANTARUM 299V 10000000000 [CFU] Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:59088-004-54 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 12/06/2023 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color shape size (solid drugs) 25 mm scoring 1 Labeler - PureTek Corporation (785961046)
Trademark Results [Bolsitol]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BOLSITOL 97939456 not registered Live/Pending |
PURETEK CORPORATION 2023-05-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.