VANFLYTA- quizartinib tablet, film coated
VANFLYTA by
Drug Labeling and Warnings
VANFLYTA by is a Prescription medication manufactured, distributed, or labeled by Daiichi Sankyo Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use VANFLYTA safely and effectively. See full prescribing information for VANFLYTA.
VANFLYTA® (quizartinib) tablets, for oral use
Initial U.S. Approval: 2023WARNING: QT PROLONGATION, TORSADES DE POINTES, and CARDIAC ARREST
See full prescribing information for complete boxed warning.
- VANFLYTA prolongs the QT interval. (12.2) Prior to VANFLYTA administration and periodically, perform electrocardiograms (ECGs), monitor for hypokalemia or hypomagnesemia, and correct deficiencies. (2.3, 5.1)
- Torsades de pointes and cardiac arrest have occurred in patients receiving VANFLYTA. Do not administer VANFLYTA to patients with severe hypokalemia, severe hypomagnesemia, or long QT syndrome. (4, 5.1)
- Do not initiate treatment with VANFLYTA or escalate the VANFLYTA dose if the QT interval corrected by Fridericia's formula (QTcF) is greater than 450 ms. (2.3, 5.1)
- Monitor ECGs more frequently if concomitant use of drugs known to prolong the QT interval is required. (2.3, 5.1)
- Reduce the VANFLYTA dose when used concomitantly with strong CYP3A inhibitors, as they may increase quizartinib exposure. (2.4, 5.1)
- VANFLYTA is available only through a restricted program called the VANFLYTA Risk Evaluation and Mitigation Strategy (REMS). (5.2)
INDICATIONS AND USAGE
VANFLYTA is a kinase inhibitor indicated in combination with standard cytarabine and anthracycline induction and cytarabine consolidation, and as maintenance monotherapy following consolidation chemotherapy, for the treatment of adult patients with newly diagnosed acute myeloid leukemia (AML) that is FLT3 internal tandem duplication (ITD)-positive as detected by an FDA-approved test. (1)
Limitations of Use:
VANFLYTA is not indicated as maintenance monotherapy following allogeneic hematopoietic stem cell transplantation (HSCT); improvement in overall survival with VANFLYTA in this setting has not been demonstrated. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Tablets: 17.7 mg or 26.5 mg. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- QT Prolongation, Torsades de Pointes, and Cardiac Arrest: Monitor electrocardiograms and levels of serum electrolytes. Reduce, interrupt, or permanently discontinue VANFLYTA as appropriate. (2.3, 5.1)
- Embryo-Fetal Toxicity: VANFLYTA can cause fetal harm. Advise females of reproductive potential and males with female partners of reproductive potential of potential risk to a fetus and to use effective contraception. (5.3, 8.1, 8.3)
ADVERSE REACTIONS
The most common (>20%) adverse reactions, including laboratory abnormalities, are lymphocytes decreased, potassium decreased, albumin decreased, phosphorus decreased, alkaline phosphatase increased, magnesium decreased, febrile neutropenia, diarrhea, mucositis, nausea, calcium decreased, abdominal pain, sepsis, neutropenia, headache, creatine phosphokinase increased, vomiting, and upper respiratory tract infection. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Daiichi Sankyo, Inc. at 1-877-437-7763 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 6/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: QT PROLONGATION, TORSADES DE POINTES, and CARDIAC ARREST
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection
2.2 Recommended Dosage
2.3 Monitoring and Dosage Modifications for Adverse Reactions
2.4 Dosage Modifications for Strong CYP3A Inhibitors
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 QT Prolongation, Torsades de Pointes, and Cardiac Arrest
5.2 VANFLYTA REMS
5.3 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: QT PROLONGATION, TORSADES DE POINTES, and CARDIAC ARREST
- VANFLYTA prolongs the QT interval in a dose- and concentration-related manner [see Clinical Pharmacology (12.2)]. Prior to VANFLYTA administration and periodically, monitor for hypokalemia or hypomagnesemia, and correct deficiencies. Perform ECGs to monitor the QTc at baseline, weekly during induction and consolidation therapy, weekly for at least the first month of maintenance, and periodically thereafter [see Dosage and Administration (2.3) and Warnings and Precautions (5.1)].
- Torsades de pointes and cardiac arrest have occurred in patients receiving VANFLYTA. Do not administer VANFLYTA to patients with severe hypokalemia, severe hypomagnesemia, or long QT syndrome [see Contraindications (4) and Warnings and Precautions (5.1)].
- Do not initiate treatment with VANFLYTA or escalate the VANFLYTA dose if the QT interval corrected by Fridericia's formula (QTcF) is greater than 450 ms [see Dosage and Administration (2.3) and Warnings and Precautions (5.1)].
- Monitor ECGs more frequently if concomitant use of drugs known to prolong the QT interval is required [see Dosage and Administration (2.3) and Warnings and Precautions (5.1)].
- Reduce the VANFLYTA dose when used concomitantly with strong CYP3A inhibitors, as they may increase quizartinib exposure [see Dosage and Administration (2.4) and Warnings and Precautions (5.1)].
- Because of the risk of QT prolongation, VANFLYTA is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the VANFLYTA REMS [see Warnings and Precautions (5.2)].
-
1 INDICATIONS AND USAGE
VANFLYTA is indicated in combination with standard cytarabine and anthracycline induction and cytarabine consolidation, and as maintenance monotherapy following consolidation chemotherapy, for the treatment of adult patients with newly diagnosed acute myeloid leukemia (AML) that is FLT3 internal tandem duplication (ITD)-positive as detected by an FDA-approved test [see Dosage and Administration (2.1) and Clinical Studies (14)].
Limitations of Use
VANFLYTA is not indicated as maintenance monotherapy following allogeneic hematopoietic stem cell transplantation (HSCT); improvement in overall survival with VANFLYTA in this setting has not been demonstrated [see Clinical Studies (14)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection
Select patients for the treatment of AML with VANFLYTA based on the presence of FLT3-ITD mutation positivity [see Clinical Studies (14)]. Information on FDA-approved tests for the detection of FLT3-ITD mutation in AML is available at: http://www.fda.gov/CompanionDiagnostics.
2.2 Recommended Dosage
- A treatment course consists of up to 2 cycles of VANFLYTA in combination with induction cytarabine and anthracycline, up to 4 cycles of VANFLYTA in combination with high-dose cytarabine consolidation, and up to 36 cycles of VANFLYTA as maintenance therapy [see Clinical Studies (14)] or until disease progression or unacceptable toxicity. VANFLYTA maintenance therapy should be initiated following consolidation chemotherapy upon blood count recovery of absolute neutrophil count >500/mm3 and platelet count >50,000/mm3.
- See Table 1 for the recommended dosage of VANFLYTA by phase of therapy.
Table 1: VANFLYTA Dosage Regimen VANFLYTA Initiation Induction* Consolidation† Maintenance Starting on Day 8
(for 7 + 3 regimen)‡Starting on Day 6 Starting on Day 1 - * Patients can receive up to 2 cycles of induction.
- † Patients can receive up to 4 cycles of consolidation.
- ‡ For 5 + 2 regimen as the second induction cycle, VANFLYTA will be given on Days 6 to 19.
Dose 35.4 mg orally once daily 35.4 mg orally once daily - Administer 26.5 mg orally once daily Days 1 through 14 of the first cycle if QTcF is less than or equal to 450 ms.
- Increase the dose to 53 mg once daily on Day 15 of the first cycle if QTcF is less than or equal to 450 ms. Maintain the 26.5 mg once daily dose if QTcF greater than 500 ms was observed during induction or consolidation.
Duration
(28-day cycles)Two weeks in each cycle (Days 8 to 21)‡ Two weeks in each cycle (Days 6 to 19) - Once daily with no break between cycles for up to 36 cycles
For patients who proceed to hematopoietic stem cell transplantation (HSCT), VANFLYTA should be stopped 7 days before the start of a conditioning regimen.
Administer VANFLYTA orally with or without food at approximately the same time each day. Swallow tablets whole. Do not cut, crush, or chew the tablets. If a dose of VANFLYTA is vomited, do not administer a replacement dose; wait until the next scheduled dose is due. If a dose of VANFLYTA is missed or not taken at the usual time, administer the dose as soon as possible on the same day and return to the usual schedule the following day. The patient should not take two doses on the same day.
2.3 Monitoring and Dosage Modifications for Adverse Reactions
Initiate VANFLYTA only if QTcF is less than or equal to 450 ms [see Warnings and Precautions (5.1)].
During induction and consolidation, perform ECGs prior to initiation and then once weekly during VANFLYTA treatment or more frequently as clinically indicated [see Warnings and Precautions (5.1)].
During maintenance, perform ECGs prior to initiation, once weekly for at least the first month following dose initiation and escalation, and thereafter as clinically indicated. Escalate the dose only if QTcF is less than or equal to 450 ms [see Dosage and Administration (2.2) and Warnings and Precautions (5.1)].
Correct electrolyte abnormalities (hypokalemia and hypomagnesemia), and if possible, avoid concomitant administration of drugs that prolong the QT interval [see Warnings and Precautions (5.1)].
For recommended dosage modifications due to adverse reactions, see Table 2. For dosage adjustments due to adverse reactions, see Table 3.
Table 2: Recommended Dosage Modifications for Adverse Reactions [see Warnings and Precautions (5.1) and Adverse Reactions (6.1)] Adverse Reaction Recommended Action Grades are in accordance with National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03 (NCI CTCAE v4.03). - * Recommend bone marrow evaluation.
QTcF between 450 ms and 480 ms (Grade 1) - Continue VANFLYTA dose.
QTcF between 481 ms and 500 ms (Grade 2) - Reduce the dose of VANFLYTA (see Table 3) without interruption.
- Resume VANFLYTA at the previous dose in the next cycle if QTcF has decreased to less than 450 ms. Monitor the patient closely for QT prolongation during the first cycle at the increased dose.
QTcF greater than 500 ms (Grade 3) - Interrupt VANFLYTA.
- Resume VANFLYTA at a reduced dose (see Table 3) when QTcF returns to less than 450 ms.
- Maintain the 26.5 mg once daily dose during maintenance if QTcF greater than 500 ms was observed during induction or consolidation.
Recurrent QTcF greater than 500 ms (Grade 3) - Permanently discontinue VANFLYTA if QTcF greater than 500 ms recurs despite appropriate dose reduction and correction/elimination of other risk factors (e.g., serum electrolyte abnormalities, concomitant QT prolonging medications).
Torsades de pointes, polymorphic ventricular tachycardia, signs/symptoms of life-threatening arrhythmia (Grade 4) - Permanently discontinue VANFLYTA.
Grade 3 or 4 non-hematologic adverse reactions - Interrupt VANFLYTA.
- Resume treatment at the previous dose if adverse reaction improves to Grade 1 or less.
- Resume treatment at a reduced dose (see Table 3) if adverse reaction improves to Grade 2.
- Discontinue if Grade 3 or 4 adverse reaction persists beyond 28 days.
Grade 3 or 4 hypokalemia (<3 mmol/L) or hypomagnesemia (<0.4 mmol/L or <0.9 mg/dL) - Interrupt VANFLYTA.
- Correct hypokalemia and hypomagnesemia according to institutional guidelines.
- VANFLYTA may be restarted at the previous dose when the adverse reaction improves to Grade 2 or less without symptoms.
Grade 4 neutropenia or thrombocytopenia after achieving remission* - Reduce VANFLYTA dose (see Table 3).
Table 3: Recommended Dosage Adjustments for Adverse Reactions for VANFLYTA Current Dosage Modified Dosage 53 mg once daily 35.4 mg once daily 35.4 mg once daily 26.5 mg once daily 26.5 mg once daily Interrupt 17.7 mg once daily Interrupt 2.4 Dosage Modifications for Strong CYP3A Inhibitors
Reduce the dosage of VANFLYTA when used concomitantly with strong CYP3A inhibitors as shown in Table 4. If the current dosage is 17.7 mg once daily, interrupt VANFLYTA treatment for the duration of strong CYP3A inhibitor use. After discontinuation of a strong CYP3A inhibitor for 5 half-lives, resume the VANFLYTA dose that was taken before initiating the strong inhibitor [see Drug Interactions (7)].
Table 4: Dosage Adjustments for Concomitant Use with Strong CYP3A Inhibitors Current Dosage Modified Dosage 53 mg once daily 26.5 mg once daily 35.4 mg once daily 17.7 mg once daily 26.5 mg once daily 17.7 mg once daily - 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
VANFLYTA is contraindicated in patients with severe hypokalemia, severe hypomagnesemia, long QT syndrome, or in patients with a history of ventricular arrhythmias or torsades de pointes [see Warnings and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 QT Prolongation, Torsades de Pointes, and Cardiac Arrest
VANFLYTA prolongs the QT interval in a dose- and concentration-dependent manner. The mechanism of QTc interval prolongation is via inhibition of the slow delayed rectifier potassium current, IKs, as compared to all other medications that prolong the QTc interval, which is via the rapid delayed rectifier potassium current, IKr. Therefore, the level of QTc prolongation with VANFLYTA that predicts the risk of cardiac arrhythmias is unclear. Inhibition of IKs and IKr may leave patients with limited reserve leading to a higher risk of QT prolongation and serious cardiac arrhythmias, including fatal outcomes [see Clinical Pharmacology (12.2)]. Torsades de pointes, ventricular fibrillation, cardiac arrest, and sudden death have occurred in patients treated with VANFLYTA.
Of the 1,081 patients with AML treated with VANFLYTA in clinical trials, torsades de pointes occurred in approximately 0.2% of patients, cardiac arrest occurred in 0.6%, including 0.4% with a fatal outcome, and 0.1% of patients experienced ventricular fibrillation [see Adverse Reactions (6.1)]. These severe cardiac arrhythmias occurred predominantly during the induction phase.
Of the 265 patients with newly diagnosed FLT3-ITD-positive AML treated with VANFLYTA in combination with chemotherapy in the clinical trial, 2.3% were found to have a QTcF greater than 500 ms and 10% of patients had an increase from baseline QTcF greater than 60 ms. The clinical trial excluded patients with a QTcF ≥450 ms or other factors that increased the risk of QT prolongation or arrhythmic events (e.g., NYHA Class III or IV congestive heart failure, hypokalemia, family history of long QT interval syndrome). Therefore, avoid use in patients who are at significant risk of developing torsades de pointes, including uncontrolled or significant cardiac disease, recent myocardial infarction, heart failure, unstable angina, bradyarrhythmias, tachyarrhythmias, uncontrolled hypertension, high-degree atrioventricular block, severe aortic stenosis, or uncontrolled hypothyroidism.
Do not initiate treatment with VANFLYTA if the QTcF interval is greater than 450 ms. Do not use VANFLYTA in patients with severe hypokalemia, severe hypomagnesemia, long QT syndrome, or in patients with a history of ventricular arrhythmias or torsades de pointes [see Contraindications (4)].
Perform an ECG and correct electrolyte abnormalities prior to initiation of treatment with VANFLYTA. During induction and consolidation, perform an ECG prior to initiation and then once weekly during VANFLYTA treatment or more frequently as clinically indicated. During maintenance, perform ECGs prior to initiation, once weekly for at least the first month following dose initiation and escalation, and as clinically indicated thereafter. Do not escalate the dose if QTcF is greater than 450 ms [see Dosage and Administration (2.3)].
Perform ECG monitoring of the QT interval more frequently in patients who are at significant risk of developing QT interval prolongation and torsades de pointes, or following dose escalation.
Monitor and correct hypokalemia and hypomagnesemia prior to and during treatment with VANFLYTA. Maintain electrolytes in the normal range. Monitor electrolytes and ECGs more frequently in patients who experience diarrhea or vomiting.
Monitor patients more frequently with ECGs if coadministration of VANFLYTA with drugs known to prolong the QT interval is required [see Drug Interactions (7)].
Reduce the VANFLYTA dose when used concomitantly with strong CYP3A inhibitors, as they may increase quizartinib exposure [see Dosage and Administration (2.4)].
Reduce VANFLYTA if QTc increases to greater than 480 ms and less than 500 ms. Interrupt and reduce VANFLYTA if QTc increases to greater than 500 ms. Permanently discontinue VANFLYTA in patients who develop recurrent QTc greater than 500 ms or QTc interval prolongation with signs or symptoms of life-threatening arrhythmia [see Dosage and Administration (2.3)].
VANFLYTA is available only through a restricted program under a REMS [see Warnings and Precautions (5.2)].
5.2 VANFLYTA REMS
VANFLYTA is available only through a restricted distribution program under a REMS called the VANFLYTA REMS because of the serious risk of QT prolongation, torsades de pointes, and cardiac arrest [see Warnings and Precautions (5.1)].
Notable requirements of the VANFLYTA REMS include the following:
- Prescribers must be certified in the VANFLYTA REMS by enrolling and completing training.
- Prescribers must counsel patients receiving VANFLYTA about the risk of QT prolongation, torsades de pointes, and cardiac arrest, and provide patients with a Patient Wallet Card.
- Pharmacies that dispense VANFLYTA must be certified with the VANFLYTA REMS and must verify prescribers are certified through the VANFLYTA REMS.
Further information about the VANFLYTA REMS is available at www.VANFLYTAREMS.com or by telephone at 1-855-212-6670.
5.3 Embryo-Fetal Toxicity
Based on findings in animals and its mechanism of action, VANFLYTA can cause fetal harm when administered to a pregnant woman. In animal reproduction studies, administration of quizartinib to pregnant rats during organogenesis at exposures 3 times the maximum recommended human dose (MRHD) of 53 mg/day caused structural abnormalities and alterations to growth.
Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with VANFLYTA and for 7 months after the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with VANFLYTA and for 4 months after the last dose [see Use in Specific Populations (8.1, 8.3)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- QT Prolongation, Torsades de Pointes, and Cardiac Arrest [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Newly Diagnosed FLT3-ITD positive AML
The safety of VANFLYTA (35.4 mg orally once daily with chemotherapy, 26.5 mg to 53 mg orally once daily as maintenance) in adult patients with newly diagnosed FLT3-ITD positive AML is based on QuANTUM-First, a randomized, double-blind clinical trial of VANFLYTA (n=265) or placebo (n=268) with chemotherapy [see Clinical Studies (14)]. Among patients who received VANFLYTA, 38% were exposed for 6 months or longer and 30% were exposed for greater than one year. On the VANFLYTA plus chemotherapy arm, 65% and 44% of patients completed induction and consolidation therapy, respectively, compared to 65% and 34% of patients in the placebo plus chemotherapy arm.
Serious adverse reactions in ≥5% of patients who received VANFLYTA plus chemotherapy were: febrile neutropenia (11%). Fatal adverse reactions occurred in 10% of patients who received VANFLYTA plus chemotherapy, including sepsis (5%), fungal infections (0.8%), brain edema (0.8%), and one case each of febrile neutropenia, pneumonia, cerebral infarction, acute respiratory distress syndrome, pulmonary embolism, ventricular dysfunction, and cardiac arrest.
Permanent discontinuation due to an adverse reaction in patients in the VANFLYTA plus chemotherapy arm occurred in 20% of patients. The most frequent (≥2%) adverse reaction which resulted in permanent discontinuation in the VANFLYTA arm was sepsis (5%).
Dosage interruptions of VANFLYTA due to an adverse reaction occurred in 34% of patients. Adverse reactions which required dosage interruption in ≥2% of patients in the VANFLYTA arm included neutropenia (11%), thrombocytopenia (5%), and myelosuppression (3%).
Dose reductions of VANFLYTA due to an adverse reaction occurred in 19% of patients. Adverse reactions which required dosage reductions in ≥2% of patients in the VANFLYTA arm were neutropenia (9%), thrombocytopenia (5%), and electrocardiogram QT prolonged (4%).
The most common adverse reactions (≥10% with a difference between arms of ≥2% compared to placebo), including laboratory abnormalities, were lymphocytes decreased, potassium decreased, albumin decreased, phosphorus decreased, alkaline phosphatase increased, magnesium decreased, febrile neutropenia, diarrhea, mucositis, nausea, calcium decreased, abdominal pain, sepsis, neutropenia, headache, creatine phosphokinase increased, vomiting, upper respiratory tract infections, hypertransaminasemia, thrombocytopenia, decreased appetite, fungal infections, epistaxis, potassium increased, herpesvirus infections, insomnia, electrocardiogram QT prolonged, magnesium increased, sodium increased, dyspepsia, anemia, and eye irritation.
Tables 5 and 6 summarize adverse reactions and laboratory abnormalities observed in patients receiving VANFLYTA in the clinical trial.
Table 5: Adverse Reactions (≥10%) in Patients with Newly Diagnosed FLT3-ITD positive AML Who Received VANFLYTA (with a Difference Between Arms of ≥2% Compared to Placebo) in the Clinical Trial Body System
Adverse ReactionVANFLYTA + Chemotherapy
(N=265)PLACEBO + Chemotherapy
(N=268)All Grades
%Grade 3 or 4
%All Grades
%Grade 3 or 4
%- * Including fatalities.
- † Includes other related terms.
- ‡ Diarrhea includes colitis, diarrhea, enteritis, enterocolitis, gastroenteritis, and neutropenic colitis.
- § Mucositis includes anal inflammation, anal ulcer, anorectal discomfort, aphthous ulcer, laryngeal inflammation, laryngeal pain, mucosal inflammation, edema mucosal, esophageal pain, esophageal ulcer, esophagitis, oral blood blister, oral disorder, oral mucosa erosion, oral mucosal blistering, oral mucosal erythema, oral pain, oropharyngeal pain, pharyngeal inflammation, proctalgia, proctitis, stomatitis, tongue ulceration, and vaginal ulceration.
- ¶ Sepsis includes acinetobacter infection, bacteremia, bacterial sepsis, corynebacterium bacteremia, device related bacteremia, device related sepsis, enterobacter sepsis, enterococcal bacteremia, enterococcal sepsis, escherichia bacteremia, escherichia sepsis, klebsiella bacteremia, klebsiella sepsis, neutropenic sepsis, pseudomonal bacteremia, pulmonary sepsis, sepsis, septic shock, staphylococcal bacteremia, staphylococcal infection, staphylococcal sepsis, stenotrophomonas sepsis, streptococcal sepsis, and streptococcal bacteremia.
- # Fungal infection includes aspergillosis oral, aspergillus infection, bronchopulmonary aspergillosis, candida infection, candida sepsis, fungal infection, fungal sepsis, fungal skin infection, fusarium infection, gastrointestinal candidiasis, hepatic infection fungal, hepatosplenic candidiasis, lower respiratory tract infection fungal, mucormycosis, oral candidiasis, oral fungal infection, oropharyngeal candidiasis, systemic candida, systemic mycosis, tinea cruris, and vulvovaginal candidiasis.
- Þ Herpesvirus infection includes disseminated varicella zoster virus infection, genital herpes, herpes simplex, herpesvirus infection, herpes zoster, oral herpes, and varicella zoster virus infection.
- ß Hypertransaminasemia includes alanine aminotransferase increased, aspartate aminotransferase increased, transaminases increased, hepatic enzymes increased, and hypertransaminasemia.
- à Eye irritation includes dry eye, eye inflammation, eye irritation, eye pain, eye pruritus, foreign body sensation in eyes, keratitis, and ulcerative keratitis.
Blood and Lymphatic System Disorders Febrile neutropenia* 44 43 42 41 Neutropenia† 29 26 14 12 Thrombocytopenia† 18 13 13 12 Anemia† 11 6 7 5 Gastrointestinal Disorders Diarrhea‡ 42 8 39 8 Mucositis§ 38 5 33 4.1 Nausea 34 1.5 31 1.9 Abdominal pain† 30 2.3 22 1.1 Vomiting 25 0 20 1.5 Dyspepsia 11 0.4 9 0.7 Infections and Infestations Sepsis¶,* 30 19 26 20 Upper respiratory tract infection† 21 2.6 12 3 Fungal infection#,* 16 6 10 3 Herpesvirus infectionÞ 14 2.6 8 1.9 Nervous System Disorders Headache† 28 0 20 0.7 Hepatobiliary disorders Hypertransaminasemiaß 19 7 14 6 Metabolism and Nutrition Disorders Decreased appetite 17 4.9 13 1.9 Respiratory, Thoracic and Mediastinal Disorders Epistaxis 15 1.1 11 0.4 Psychiatric Disorders Insomnia 14 0 11 0 Investigations Electrocardiogram QT prolonged† 14 3 4.1 1.1 Eye Disorders Eye irritationà 11 0 7 0 Laboratory Abnormalities
Prolonged thrombocytopenia or neutropenia in the absence of active leukemia lasting past cycle day 42 of induction cycle 1 were noted in 8% of patients on the VANFLYTA plus chemotherapy arm and 4% of patients in the placebo plus chemotherapy arm.
Table 6: Select Laboratory Abnormalities (≥10%) That Worsened from Baseline in Patients with Newly Diagnosed FLT3-ITD positive AML (with a Difference Between Arms of ≥2% Compared to Placebo) in the Clinical Trial Laboratory Abnormality VANFLYTA + Chemotherapy* PLACEBO + Chemotherapy* All Grades% Grades 3 or 4% All Grades% Grades 3 or 4% - * The denominator used to calculate the rate varied from 199 to 260 in VANFLYTA + Chemotherapy and from 187 to 267 in PLACEBO + Chemotherapy based on the number of patients with a baseline value and at least one post-treatment value.
Lymphocytes decreased 60 57 55 51 Potassium decreased 59 22 56 18 Albumin decreased 53 1.6 45 4.3 Phosphorus decreased 52 22 48 19 Alkaline phosphatase increased 51 1.6 47 1.9 Magnesium decreased 44 2 42 1.1 Calcium decreased 33 2.4 27 1.6 Creatine phosphokinase increased 26 2.5 7 0.5 Potassium increased 15 1.2 11 0.8 Magnesium increased 14 2.8 9 1.2 Sodium increased 13 0 10 0.4 -
7 DRUG INTERACTIONS
Table 7: Effect of Other Drugs on VANFLYTA Strong CYP3A Inhibitors Clinical Impact VANFLYTA is a CYP3A substrate. Concomitant use of VANFLYTA with a strong CYP3A inhibitor increases quizartinib systemic exposure [see Clinical Pharmacology (12.3)], which may increase the risk of VANFLYTA adverse reactions. Prevention or Management Reduce the dosage of VANFLYTA [see Dosage and Administration (2.4)]. Strong or Moderate CYP3A Inducers Clinical Impact Concomitant use of VANFLYTA with strong or moderate CYP3A inducers decreases quizartinib systemic exposure [see Clinical Pharmacology (12.3)], which may reduce VANFLYTA efficacy. Prevention or Management Avoid concomitant use of VANFLYTA with strong or moderate CYP3A inducers [see Clinical Pharmacology (12.3)]. QT Interval Prolonging Drugs Clinical Impact VANFLYTA prolongs the QT/QTc interval.
Coadministration of VANFLYTA with other drugs that prolong the QT interval may further increase the incidence of QT prolongation [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.2)].Prevention or Management Monitor patients more frequently with ECG if coadministration of VANFLYTA with drugs known to prolong the QT interval is required. Examples of QT prolonging drugs include but are not limited to antifungal azoles, ondansetron, granisetron, azithromycin, pentamidine, doxycycline, moxifloxacin, atovaquone, prochlorperazine, and tacrolimus. -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings from animal studies and its mechanism of action, VANFLYTA can cause embryo-fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)].
There are no available data on VANFLYTA use in pregnant women to evaluate for a drug-associated risk. In animal reproduction studies, oral administration of quizartinib to pregnant rats during organogenesis resulted in adverse developmental outcomes including structural abnormalities and alterations to growth at maternal exposures approximately 3 times those in patients at the maximum recommended human dose (MRHD) of 53 mg/day (see Data). Advise pregnant women of the potential risk to a fetus.
The background risk in the U.S. general population of major birth defects is 2-4%, and of miscarriage is 15-20% of clinically recognized pregnancies.
Data
Animal Data
In an embryo-fetal development study in rats, pregnant animals received oral doses of quizartinib of 0, 0.6, 2, or 6 mg/kg/day during the period of organogenesis. Administration of quizartinib at the dose of 6 mg/kg/day was associated with adverse developmental outcomes including structural abnormalities (anasarca and edema) and alterations to growth (lower fetal weights and effects on skeletal ossification). At this dose, the maternal systemic exposures (AUC) were approximately 3 times the human exposure at the MRHD of 53 mg/day.
8.2 Lactation
Risk Summary
There are no data on the presence of quizartinib or its metabolites in human milk, or the effects on the breastfed child or milk production. Because of the potential for serious adverse reactions in a breastfed child, advise women not to breastfeed during treatment with VANFLYTA and for one month after the last dose.
8.3 Females and Males of Reproductive Potential
VANFLYTA can cause embryo-fetal harm when administered to pregnant women [see Use in Specific Populations (8.1)].
Pregnancy Testing
Verify pregnancy status in females of reproductive potential within seven days before starting treatment with VANFLYTA.
Contraception
Females
Advise female patients of reproductive potential to use effective contraception during treatment with VANFLYTA and for 7 months after the last dose.
Males
Based on genotoxicity findings, advise male patients with female partners of reproductive potential to use effective contraception during treatment with VANFLYTA and for 4 months after the last dose [see Nonclinical Toxicology (13.1)].
Infertility
Females
Based on findings from animal studies, VANFLYTA may impair female fertility. These effects on fertility were reversible [see Nonclinical Toxicology (13.1)].
Males
Based on findings from animal studies, VANFLYTA may impair male fertility. These effects on fertility were reversible [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
Safety and effectiveness of VANFLYTA have not been established in pediatric patients.
8.5 Geriatric Use
There were 533 patients with newly diagnosed AML in the clinical study; of the total number of VANFLYTA-treated patients, 69 (26%) were 65 years of age and older, while 1 (0.4%) was 75 years of age [see Clinical Studies (14)]. No overall differences in safety or efficacy were observed between patients 65 years of age and older and younger adult patients.
8.6 Renal Impairment
No dosage adjustment is recommended in patients with mild to moderate renal impairment (i.e., estimated creatinine clearance [CLcr] by Cockcroft-Gault equation: CLcr 30 to 89 mL/min). VANFLYTA has not been studied in patients with severe renal impairment (CLcr <30 mL/min) [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
No dosage adjustment is recommended in patients with mild hepatic impairment (Child-Pugh Class A or total bilirubin ≤ upper limit of normal [ULN] and aspartate aminotransferase [AST] >ULN, or total bilirubin >1 to 1.5 times ULN and any value for AST) or moderate hepatic impairment (Child-Pugh Class B or total bilirubin >1.5 to 3 times ULN and any value for AST). VANFLYTA has not been studied in patients with severe (Child-Pugh Class C or total bilirubin >3 times ULN and any value for AST) hepatic impairment [see Clinical Pharmacology (12.3)].
-
11 DESCRIPTION
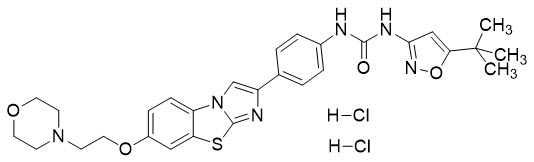
VANFLYTA (quizartinib) is a kinase inhibitor for oral use. The chemical name of quizartinib dihydrochloride is 1-(5-tert-butyl-1,2-oxazol-3-yl)-3-(4-{7-[2-(morpholin-4-yl)ethoxy]imidazo[2,1-b][1,3]benzothiazol-2-yl}phenyl)urea dihydrochloride. Quizartinib dihydrochloride is a white to off-white solid with a molecular formula of C29H32N6O4S∙2 HCl and a molecular weight of 633.6 for the salt and 560.7 for the free base. The aqueous solubility of quizartinib dihydrochloride (pKa 4.75 and 3.16) decreases with increasing pH. It is very slightly soluble in aqueous media at pH 1 and practically insoluble or insoluble at pH 2 and higher. Quizartinib dihydrochloride is very slightly soluble in ethanol. The chemical structure of quizartinib dihydrochloride is:
VANFLYTA is supplied as film-coated tablets containing 17.7 mg or 26.5 mg of quizartinib, which are equivalent to 20 mg and 30 mg quizartinib dihydrochloride, respectively. The inactive ingredients in the tablet core are hydroxypropyl betadex, microcrystalline cellulose, and magnesium stearate. The tablet coating consists of hypromellose, talc, triacetin, and titanium dioxide. The 26.5 mg tablet coating also contains ferric oxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Quizartinib is a small molecule inhibitor of the receptor tyrosine kinase FLT3. Quizartinib and its major active metabolite AC886 bind to the adenosine triphosphate (ATP) binding domain of FLT3 with comparable affinity, and both had 10-fold lower affinity towards FLT3-ITD mutation compared to FLT3 in a binding assay. Quizartinib and AC886 inhibited FLT3 kinase activity, preventing autophosphorylation of the receptor, thereby inhibiting downstream FLT3 receptor signaling and blocking FLT3-ITD-dependent cell proliferation. Quizartinib showed antitumor activity in a mouse model of FLT3-ITD-dependent leukemia.
12.2 Pharmacodynamics
The exposure-response relationship and time course of pharmacodynamic response for the safety and effectiveness of quizartinib have not been fully characterized.
Cardiac Electrophysiology
In vitro studies have shown that quizartinib is a predominant inhibitor of the slow delayed rectifier potassium current, IKs.
The exposure-response analysis predicted a concentration-dependent QTcF interval median prolongation of 18 and 24 ms [upper bound of 2-sided 90% confidence interval (CI): 21 and 27 ms] at the median steady-state Cmax of quizartinib at the 26.5 mg and 53 mg dose level during maintenance therapy [see Warnings and Precautions (5.1) and Drug Interactions (7)].
12.3 Pharmacokinetics
The pharmacokinetics of quizartinib and its major circulating active metabolite (AC886) were characterized in healthy subjects and in patients with cancer and are presented as geometric mean (percent coefficient of variation [%CV]) unless otherwise specified.
Steady state quizartinib concentrations were achieved at Day 15 following once daily dosage in patients with AML. Quizartinib exposure (AUC and Cmax) increased proportionally in the dose range of 26.5 mg (0.75 times the recommended 35.4 mg induction dose) to 79.5 mg (2.25 times the recommended 35.4 mg induction dose) in healthy subjects and 17.7 mg (0.5 times the recommended 35.4 mg induction dose) to 53 mg (1.5 times the recommended 35.4 mg induction dose) in patients with AML.
Table 8: Pharmacokinetic Properties of Quizartinib and AC886 Quizartinib AC886 - * Mean (±SD)
General Information Steady state exposure following 35.4 mg VANFLYTA once daily during induction therapy in patients with newly diagnosed AML Cmax 140 ng/mL (71%) 163 ng/mL (52%) AUC0-24h 2,680 ng∙h/mL (85%) 3,590 ng∙h/mL (51%) Steady state exposure following 35.4 mg VANFLYTA once daily during consolidation therapy in patients with newly diagnosed AML Cmax 204 ng/mL (64%) 172 ng/mL (47%) AUC0-24h 3,930 ng∙h/mL (78%) 3,800 ng∙h/mL (46%) Steady state exposure following 53 mg VANFLYTA once daily during maintenance therapy in patients with newly diagnosed AML Cmax 529 ng/mL (60%) 262 ng/mL (48%) AUC0-24h 10,200 ng∙h/mL (75%) 5,790 ng∙h/mL (46%) Accumulation ratio*
(AUC0-24h)5.4 (4.4) 8.7 (6.8) Absorption
The mean (SD) absolute bioavailability of quizartinib from the tablet formulation was 71% (±7%) in healthy subjects. After oral administration under fasted conditions, time to peak concentration (median Tmax) of quizartinib and AC886 measured post dose was approximately 4 hours (range 2 to 8 hours) and 5 to 6 hours (range 4 to 120 hours), respectively, in healthy subjects.
No clinically significant differences in the pharmacokinetics of quizartinib were observed when administered with a high-fat, high-calorie meal.
Distribution
Volume of distribution at steady state in healthy subjects was estimated to be 275 L (17%).
In vitro plasma protein binding of quizartinib and AC886 is 99% or greater.
In vitro blood-to-plasma ratio for quizartinib and AC886 ranges from 0.79-1.30 and 1.36-3.19, respectively.
Elimination
Total body clearance of quizartinib in healthy subjects was estimated to be 2.23 L/hour (29%).
The mean (SD) effective half-lives (t1/2) in patients with newly diagnosed AML for quizartinib and AC886 during maintenance therapy are 81 hours (±73) and 136 hours (±113), respectively.
Specific Populations
There were no clinically significant differences in the exposure of quizartinib and AC886 based on age (range 18 to 91 years), sex, race (White 65%, Asian 18%, Black 9%), or body weight (range 37 to 153 kg).
Patients with Renal Impairment
Mild or moderate renal impairment (i.e., estimated creatinine clearance [CLcr] by Cockcroft-Gault equation: 30 to 89 mL/min) were not associated with clinically significant differences in the exposure of quizartinib and AC886.
The impact of severe renal impairment (CLcr <30 mL/min) on the pharmacokinetics of quizartinib and AC886 is unknown [see Use in Specific Populations (8.6)].
Patients with Hepatic Impairment
Mild (total bilirubin ≤ upper limit of normal [ULN] and aspartate aminotransferase [AST] >ULN or total bilirubin >1 to 1.5 times ULN and any value for AST) or moderate (total bilirubin >1.5 to 3 times ULN and any value for AST) hepatic impairment were not associated with clinically significant differences in the exposure of quizartinib and AC886.
The impact of severe hepatic impairment (Child-Pugh Class C or total bilirubin >3 times ULN and any value for AST) on the pharmacokinetics of quizartinib and AC886 is unknown [see Use in Specific Populations (8.7)].
Drug Interaction Studies and Model-Informed Approaches
Clinical Studies
Strong CYP3A Inhibitors
The AUC of quizartinib increased by 94% and the Cmax by 17% following coadministration of a single 53 mg quizartinib dose with ketoconazole (a strong CYP3A inhibitor). The AUCinf of AC886 decreased by 94% and Cmax by 60%.
Moderate CYP3A Inhibitors
A clinically significant change in quizartinib and AC886 exposure (Cmax and AUCinf) was not observed following coadministration of a single quizartinib dose with fluconazole (a moderate CYP3A inhibitor).
Strong or Moderate CYP3A Inducers
The AUCinf of quizartinib decreased by 90% and Cmax by 45% following concomitant use of a single 53 mg dose of quizartinib with efavirenz (a moderate CYP3A inducer). The AUC of AC886 decreased by 96% and the Cmax by 68%. The effect of concomitant use with a strong CYP3A inducer may result in even greater effect on quizartinib pharmacokinetics based on mechanistic understanding of the drugs involved.
In Vitro Studies
Cytochrome P450 (CYP) Enzymes
Quizartinib does not inhibit CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, or CYP3A4. Quizartinib does not induce CYP3A4, CYP1A2, or CYP2B6.
AC886 does not inhibit CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2D6, CYP2C19, or CYP3A4. AC886 does not induce CYP3A4, CYP1A2, or CYP2B6.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been conducted with quizartinib.
Quizartinib was mutagenic in a bacterial reverse mutation (Ames) assay and not mutagenic in an in vivo transgenic rat mutation assay. Quizartinib was not genotoxic in vitro in mouse lymphoma thymidine kinase mutation and human lymphocyte chromosome aberration assays, or in an in vivo rat bone marrow micronucleus assay.
Fertility studies in animals have not been conducted with quizartinib. However, adverse findings in male and female reproductive systems were observed in repeat-dose toxicity studies in rats and monkeys. Findings in female animals (rats or monkeys) included ovarian cysts, vaginal mucosal modifications, and atrophy of the uterus, ovary, and vagina, starting at exposures (AUC) approximately 0.2 times the MRHD of 53 mg/day. In male animals (rats and monkeys), findings included testicular seminiferous tubular degeneration, failure of sperm release, germ cell depletion in the testes, and oligospermia/aspermia, starting at exposures approximately 0.4 times the MRHD. After approximately one month of recovery period, all these findings except the vaginal mucosal modifications in the female rats were reversible.
-
14 CLINICAL STUDIES
The efficacy of VANFLYTA in combination with chemotherapy was evaluated in QuANTUM-First (NCT02668653), a randomized, double-blind, placebo-controlled study of 539 patients with newly diagnosed FLT3-ITD positive AML. FLT3-ITD status was determined prospectively with a clinical trial assay and verified retrospectively using the companion diagnostic LeukoStrat® CDx FLT3 Mutation Assay, which is an FDA-approved test for selection of patients with AML for VANFLYTA treatment.
Patients were stratified by age (<60 versus ≥60 years), white blood cell count at diagnosis (<40×109/L versus ≥40×109/L), and region (North America, Europe versus Asia, other regions). Patients were randomized (1:1) to receive VANFLYTA (n=268) or placebo (n=271) in combination with induction and consolidation therapy and as maintenance monotherapy according to the initial assignment, as follows: a) Induction: 35.4 mg orally once daily on Days 8-21 of 7 + 3 (cytarabine [100 or 200 mg/m2/day] on Days 1 to 7 plus daunorubicin [60 mg/m2/day] or idarubicin [12 mg/m2/day] on Days 1 to 3) and on Days 8-21 or 6-19 of an optional second induction (7 + 3 or 5 + 2 [5 days cytarabine plus 2 days daunorubicin or idarubicin], respectively), b) Consolidation: 35.4 mg orally once daily on Days 6-19 of high-dose cytarabine (1.5 to 3 g/m2 every 12 hours on Days 1, 3 and 5) for up to 4 cycles, and c) Maintenance: 26.5 mg orally once daily on Days 1 to 14 and 53 mg once daily thereafter for up to thirty-six 28-day cycles. There was no re-randomization at the start of post consolidation therapy. Patients who proceeded to hematopoietic stem cell transplantation (HSCT) initiated maintenance therapy after recovery from the HSCT.
The two treatment groups were generally balanced with respect to baseline demographics and disease characteristics. Of the 539 randomized patients, the median age was 56 years (range 20-75 years); 46% were male; 60% were White, 29% were Asian, 1% were Black, and 10% were other races. Eighty-four percent had an Eastern Cooperative Oncology Group (ECOG) baseline performance status of 0 or 1. The majority of the patients (72%) had intermediate risk cytogenetics at baseline. FLT3-ITD variant allelic frequency (VAF) was 3-25% in 36% of patients, >25-50% in 52% of patients, and >50% in 12% of patients. NPM1 mutations were identified in 52% of patients.
A second course of induction was administered to 20% of the patients, 65% initiated at least one cycle of consolidation, and 39% initiated maintenance. Among the patients who entered maintenance, 64% completed at least 12 cycles, 36% completed at least 24 cycles, and 16% completed all 36 planned cycles of maintenance. Twenty-nine percent (157/539) of the patients underwent HSCT in first complete remission (CR). The overall rate of HSCT (including the following settings: first CR, induction failure, or salvage after relapse) was 54% (144/268) in the VANFLYTA plus standard chemotherapy arm versus 47% (128/271) in the placebo plus standard chemotherapy arm. All patients were followed for survival.
Efficacy was established on the basis of overall survival (OS), measured from the date of randomization until death by any cause. The primary analysis was conducted after a minimum follow-up of 24 months after the randomization of the last patient. The study demonstrated a statistically significant improvement in OS for the VANFLYTA arm [hazard ratio (HR) 0.78; 95% CI: 0.62, 0.98; 2-sided p=0.0324] (see Figure 1).
Figure 1: Kaplan-Meier Curve for Overall Survival in QuANTUM-First 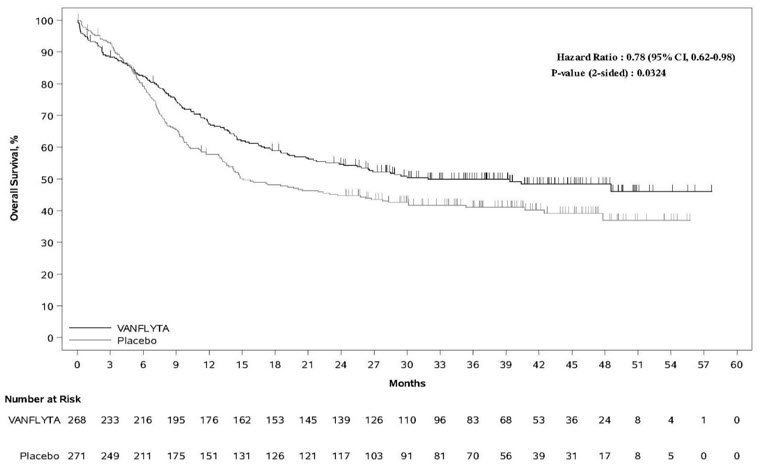
In an exploratory subgroup analysis of the 89/208 (43%) of patients who received maintenance therapy with VANFLYTA or placebo following consolidation chemotherapy, the OS HR was 0.40 (95% CI: 0.19, 0.84). Of 119/208 (57%) of patients who received maintenance therapy with VANFLYTA or placebo following HSCT, the OS HR was 1.62 (95% CI: 0.62, 4.22).
The CR rate in the VANFLYTA arm was 55% (95% CI: 48.7, 60.9) with a median duration of CR of 38.6 months (95% CI: 21.9, NE), and the CR rate in the placebo arm was 55% (95% CI: 49.2, 61.4) with a median duration of CR of 12.4 months (95% CI: 8.8, 22.7).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
VANFLYTA (quizartinib) is supplied as round, film-coated tablets, packaged in bottles with a child-resistant closure.
Store at 20°C to 25°C (68°F to 77°F); excursions permitted from 15°C to 30°C (59°F to 86°F) [See USP Controlled Room Temperature].
Tablet Strength Tablet Description Package Configuration NDC 17.7 mg White, round, film-coated, debossed with "DSC511", 28-count bottle 65597-504-28 14-count bottle 65597-504-04 26.5 mg Yellow, round, film-coated, debossed with "DSC512" 28-count bottle 65597-511-28 14-count bottle 65597-511-04 -
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
QT Prolongation, Torsades de Pointes, and Cardiac Arrest
Inform patients of symptoms that may be associated with significant QTc interval prolongation including dizziness, lightheadedness, and fainting. Advise patients to report these symptoms and the use of all medications to their healthcare provider [see Warnings and Precautions (5.1)].
VANFLYTA REMS
VANFLYTA is available only through a restricted program called the VANFLYTA REMS. Inform patients that they will be given a VANFLYTA Patient Wallet Card that they should carry with them at all times and show to all of their healthcare providers. This card describes signs and symptoms related to QT prolongation/cardiac arrhythmia which, if experienced, should prompt the patient to immediately seek medical attention [see Warnings and Precautions (5.2)].
Drug Interactions
Advise patients to inform their healthcare providers of all concomitant medications, including over-the-counter medications, vitamins, and herbal products. Advise patients to avoid concomitant use of St. John's wort as it is a strong CYP3A inducer [see Drug Interactions (7)].
Embryo-Fetal Toxicity and Use of Contraceptives
Advise pregnant women of the potential risk to the fetus. Advise female patients of reproductive potential to use effective contraception during treatment with VANFLYTA and for 7 months after the last dose. Advise patients to notify their healthcare provider immediately in the event of a pregnancy or if pregnancy is suspected during VANFLYTA treatment. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with VANFLYTA and for 4 months after the last dose [see Warnings and Precautions (5.3) and Use in Specific Populations (8.1, 8.3)].
Infertility
Advise females and males of reproductive potential that VANFLYTA may impair fertility [see Use in Specific Populations (8.3) and Nonclinical Toxicology (13.1)].
Lactation
Advise women not to breastfeed during treatment with VANFLYTA and for one month after the last dose [see Use in Specific Populations (8.2)].
Dosing and Storage Instructions
- Advise patients that VANFLYTA should be taken once daily at approximately the same time each day and may be taken with or without food.
- Advise patients to swallow tablets whole. Advise patients not to cut, crush, or chew the tablets [see Dosage and Administration (2.2)].
- Instruct patients that if a dose of VANFLYTA is vomited, not to take an additional dose that day, and to wait until the next scheduled dose on the following day. If a dose of VANFLYTA is missed or not taken at the usual time, instruct patients to take the dose as soon as possible on the same day and return to the usual dosing schedule the following day.
- Store VANFLYTA at room temperature from 20°C to 25°C (68°F to 77°F).
- SPL UNCLASSIFIED SECTION
-
MEDICATION GUIDE
MEDICATION GUIDE
VANFLYTA (van-FLITT-ah)
(quizartinib)
tabletsThis Medication Guide has been approved by the U.S. Food and Drug Administration. Issued: 7/2023 What is the most important information I should know about VANFLYTA?
VANFLYTA may cause serious side effects, including:- Changes in the electrical activity of your heart called QT prolongation, torsades de pointes, and your heart stopping (cardiac arrest). QT prolongation can cause irregular heartbeats that can be life-threatening or lead to death. Your healthcare provider will check the electrical activity of your heart with a test called an electrocardiogram (ECG) and will also do blood tests to check your potassium and magnesium levels before and during treatment with VANFLYTA. Tell your healthcare provider right away if you have an irregular heartbeat or feel dizzy, lightheaded, or faint, or have diarrhea or vomiting.
You will receive a VANFLYTA Patient Wallet Card from your healthcare provider. Carry the VANFLYTA Patient Wallet Card with you at all times and show it to all of your healthcare providers. The VANFLYTA Patient Wallet Card lists signs and symptoms of QT prolongation and torsades de pointes.
Get medical help right away if you develop any of the signs and symptoms listed on the VANFLYTA Patient Wallet Card. You may need to be treated in a hospital.
See "What are the possible side effects of VANFLYTA?" for more information about side effects.What is VANFLYTA?
VANFLYTA is a prescription medicine used in combination with certain chemotherapy medicines and alone as maintenance therapy to treat adults with newly diagnosed acute myeloid leukemia (AML) with a FLT3-ITD mutation.
Your healthcare provider will perform a test to make sure that VANFLYTA is right for you.
VANFLYTA is not for use alone as maintenance therapy after a hematopoietic stem cell transplant.
It is not known if VANFLYTA is safe and effective in children.Do not take VANFLYTA if you have very low potassium, very low magnesium, long QT syndrome, or a history of ventricular arrhythmias or torsades de pointes. Before you take VANFLYTA, tell your healthcare provider about all of your medical conditions, including if you: - have any heart problems.
- have low blood levels of potassium or magnesium.
- are pregnant or plan to become pregnant. VANFLYTA can harm your unborn baby. Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with VANFLYTA.
- If you are able to become pregnant, your healthcare provider will perform a pregnancy test within 7 days before you start treatment with VANFLYTA.
- Females who are able to become pregnant should use effective birth control (contraception) during treatment with VANFLYTA and for 7 months after the last dose of VANFLYTA.
- Males who have female partners who are able to become pregnant should use effective birth control during treatment with VANFLYTA and for 4 months after the last dose of VANFLYTA.
- Talk to your healthcare provider about birth control methods you can use during this time.
- are breastfeeding or plan to breastfeed. It is not known if VANFLYTA passes into your breast milk. Do not breastfeed during treatment with VANFLYTA and for 1 month after the last dose of VANFLYTA.
Especially tell your healthcare provider if you take St. John's wort. You should not take St. John's wort during treatment with VANFLYTA.How should I take VANFLYTA? - Take VANFLYTA exactly as your healthcare provider tells you to. Do not change your dose or stop taking VANFLYTA unless your healthcare provider tells you to.
- Take VANFLYTA by mouth 1 time a day at about the same time each day.
- Take VANFLYTA with or without food.
- Swallow VANFLYTA tablets whole. Do not cut, crush, or chew the tablets.
- If you miss a dose of VANFLYTA or did not take it at your usual time, take your dose as soon as possible on the same day. Take your next dose at your usual time on the next day. Do not take 2 doses on the same day to make up for a missed dose.
- If you vomit after taking a dose of VANFLYTA, do not take another dose. Take your next dose at your usual time the next day.
What are the possible side effects of VANFLYTA?
VANFLYTA may cause serious side effects, including: The most common side effects of VANFLYTA include:- low white blood cell counts
- changes in levels of electrolytes in the blood
- changes in liver function tests
- low white blood cell counts with fever
- diarrhea
- mouth sores
- nausea
- stomach (abdominal) pain
- serious infection throughout the body and organs (sepsis)
- headache
- vomiting
- upper respiratory tract infections
- low platelet counts
- decreased appetite
- fungal infections
- nosebleed
- herpesvirus infections
- trouble sleeping
- abnormal electrocardiogram (QT prolongation)
- upset stomach
- low red blood cell counts (anemia)
- eye irritation
Your healthcare provider will do blood tests and ECGs before you start and during treatment with VANFLYTA. Your healthcare provider may tell you to decrease your dose, temporarily stop, or permanently stop taking VANFLYTA if you develop certain side effects during treatment with VANFLYTA.
VANFLYTA may cause fertility problems in females and males, which may affect your ability to have children. Talk to your healthcare provider if you have concerns about fertility.
These are not all the possible side effects of VANFLYTA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store VANFLYTA? - Store VANFLYTA at room temperature between 68°F and 77°F (20°C and 25°C).
General information about the safe and effective use of VANFLYTA.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use VANFLYTA for a condition for which it is not prescribed. Do not give VANFLYTA to other people even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for information about VANFLYTA that is written for health professionals.What are the ingredients in VANFLYTA?
Active ingredient: quizartinib
Inactive ingredients:- Tablet core: hydroxypropyl betadex, microcrystalline cellulose, and magnesium stearate.
- Tablet coating: hypromellose, talc, triacetin, and titanium dioxide. The 26.5 mg tablet coating also contains ferric oxide.
VANFLYTA® is a registered trademark of Daiichi Sankyo Company, Ltd.
Copyright © 2023, Daiichi Sankyo, Inc.
USMG-VAN-C1-0723-r001
For more information about VANFLYTA, ask your healthcare provider or pharmacist, visit http://www.vanflyta.com, or call 1-877-437-7763. -
PRINCIPAL DISPLAY PANEL - 17.7 mg Tablet Bottle Carton
NDC: 65597-504-04
14 tabletsVANFLYTA®
(quizartinib) tablets17.7 mg
Rx only
Swallow tablets whole. Do not cut,
crush, or chew the tablets.Dispense accompanying
Medication Guide to each patient.Daiichi-Sankyo
-
PRINCIPAL DISPLAY PANEL - 26.5 mg Tablet Bottle Carton
NDC: 65597-511-04
14 tabletsVANFLYTA®
(quizartinib) tablets26.5 mg
Rx only
Swallow tablets whole. Do not cut,
crush, or chew the tablets.Dispense accompanying
Medication Guide to each patient.Daiichi-Sankyo
-
INGREDIENTS AND APPEARANCE
VANFLYTA
quizartinib tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 65597-504 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength QUIZARTINIB DIHYDROCHLORIDE (UNII: WK7Q6ZIZ10) (QUIZARTINIB - UNII:7LA4O6Q0D3) QUIZARTINIB 17.7 mg Inactive Ingredients Ingredient Name Strength HYDROXYPROPYL BETADEX (UNII: 1I96OHX6EK) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) TALC (UNII: 7SEV7J4R1U) TRIACETIN (UNII: XHX3C3X673) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE Score no score Shape ROUND Size 9mm Flavor Imprint Code DSC;511 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65597-504-04 1 in 1 CARTON 07/20/2023 1 14 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC: 65597-504-28 1 in 1 CARTON 07/20/2023 2 28 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216993 07/20/2023 VANFLYTA
quizartinib tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 65597-511 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength QUIZARTINIB DIHYDROCHLORIDE (UNII: WK7Q6ZIZ10) (QUIZARTINIB - UNII:7LA4O6Q0D3) QUIZARTINIB 26.5 mg Inactive Ingredients Ingredient Name Strength HYDROXYPROPYL BETADEX (UNII: 1I96OHX6EK) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) TALC (UNII: 7SEV7J4R1U) TRIACETIN (UNII: XHX3C3X673) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color yellow Score no score Shape ROUND Size 10mm Flavor Imprint Code DSC;512 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65597-511-04 1 in 1 CARTON 07/20/2023 1 14 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC: 65597-511-28 1 in 1 CARTON 07/20/2023 2 28 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216993 07/20/2023 Labeler - Daiichi Sankyo Inc. (068605067)
Trademark Results [VANFLYTA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VANFLYTA 97244511 not registered Live/Pending |
DAIICHI SANKYO COMPANY, LIMITED 2022-01-28 |
 VANFLYTA 79188048 5066600 Live/Registered |
DAIICHI SANKYO COMPANY, LIMITED 2016-02-25 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.