PHENOBARBITAL TABLETS - CA1 CIV
Phenobarbital by
Drug Labeling and Warnings
Phenobarbital by is a Animal medication manufactured, distributed, or labeled by Taro Pharmaceuticals U.S.A. Inc., Genus Lifesciences Inc., Sunrise Pharmaceutical Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
PHENOBARBITAL- phenobarbital tablet
Sun Pharmaceutical Industries, Inc.
----------
PHENOBARBITAL TABLETS - CA1 CIV
Antiepileptic for use in dogs only.
Conditionally approved by FDA pending a full demonstration of effectiveness under application number 141-578.
It is a violation of Federal law to use this product in a manner other than directed on the labeling.
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian. Use only as directed.
DESCRIPTION:
PHENOBARBITAL TABLETS-CA1 is an unflavored, bisected tablet that contains phenobarbital. Phenobarbital is white, odorless, small crystals or white crystalline powder with a bitter taste. The molar mass of phenobarbital is 232.235 g/mol, with solubility in water, alcohol, ether, and in fixed alkali hydroxides and carbonates.
INDICATION:
PHENOBARBITAL TABLETS-CA1 are indicated for the control of seizures associated with idiopathic epilepsy in dogs.
DOSAGE AND ADMINISTRATION:
PHENOBARBITAL TABLETS-CA1 is given orally twice a day at the minimum dosage of 2.5 mg/kg and may be titrated to effect to a maximum dosage of 5 mg/kg. The dosage of PHENOBARBITAL TABLETS-CA1 should be adjusted based on monitoring the clinical response of the individual patient. PHENOBARBITAL TABLETS-CA1 should be dosed with food.
All split tablets should be used within 90 days.
CONTRAINDICATIONS:
PHENOBARBITAL TABLETS-CA1 should not be used in animals with a history of hypersensitivity to phenobarbital.
WARNINGS:
Human Safety Warnings
Not for use in humans. Keep this and all medications out of reach of children.
Because phenobarbital may cause developmental effects, women who are pregnant or may become pregnant should handle and administer PHENOBARBITAL TABLETS-CA1 with caution.
Human User Safety While Handling PHENOBARBITAL TABLETS-CA1
Drug Abuse, Addiction, and Diversion
Controlled Substance:
PHENOBARBITAL TABLETS-CA1 contains phenobarbital, a Schedule IV controlled substance.
Abuse: Phenobarbital is a barbiturate and a central nervous system (CNS) depressant with a potential for misuse, abuse, and addiction. Abuse is defined as the intentional, non-therapeutic use of a drug, even once, to achieve a desired psychological or physiological effect. Misuse is the intentional use, for therapeutic purposes, of a drug by an individual in a way other than prescribed by a health care provider. Drug addiction is a cluster of behavioral, cognitive, and physiological phenomena that may include a strong desire to take the drug, difficulties in controlling drug use (e.g., continuing drug use despite harmful consequences, giving a higher priority to drug use than other activities and obligations), and possible tolerance or physical dependence.
Abuse and misuse of barbiturates often (but not always) involve the use of doses exceeding the doses used in clinical practice and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death. Barbiturates, including phenobarbital, are often sought by individuals who abuse drugs and other substances, and by individuals with addictive disorders.
Symptoms of acute intoxication with barbiturates include unsteady gait, slurred speech, and sustained nystagmus. Mental signs of chronic intoxication include confusion, poor judgment, irritability, insomnia, and somatic complaints. The use of phenobarbital alone can result in death; however, death is more often associated with phenobarbital use in the context of polysubstance use (especially with other CNS depressants, such as opioids and alcohol).
Phenobarbital may produce physical dependence. Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or a significant dose reduction of a drug. Abrupt discontinuation or rapid dosage reduction of phenobarbital may precipitate acute withdrawal reactions, including seizures, which can be life-threatening.
Tolerance may occur with continued use. As tolerance to barbiturates develops, the amount needed to maintain the same level of intoxication increases; tolerance to a fatal dosage, however, does not increase more than twofold. As this occurs, the margin between intoxicating dosage and fatal dosage becomes smaller.
Consider the potential risks of misuse or abuse before prescribing this product. Signs of phenobarbital misuse or abuse include drug seeking behavior.
Diversion: PHENOBARBITAL TABLETS-CA1 is a Schedule IV antiepileptic and should be handled appropriately to minimize the risk of diversion, including restriction of access, the use of accounting procedures, and proper disposal methods. Store in a locked cabinet according to federal and state controlled substance requirements and guidelines. Any unused or expired bottles must be destroyed by a reverse distributor. For further information, contact your local DEA field office or call Genus Lifesciences Inc. at 1-833-599-7582.
Note to physician: PHENOBARBITAL TABLETS-CA1 contains phenobarbital.
Users can call Genus Lifesciences Inc. at 1-833-599-7582 at any time for product safety support. To obtain a Safety Data Sheet(s), contact Genus Lifesciences Inc. at 1-833-599-7582 or at https://www.genuslifesciences.com.
Animal Safety Warnings
Not for use in cats.
Do not use in pregnant or lactating dogs. Based on data from laboratory animals, administration of PHENOBARBITAL TABLETS-CA1 to pregnant dogs may produce congenital malformations in the developing fetus. Phenobarbital may be present and accumulate in the milk of lactating dogs.
Keep PHENOBARBITAL TABLETS-CA1 in a secure location out of the reach of dogs, cats, or other animals to prevent accidental ingestion or overdose.
PRECAUTIONS:
PHENOBARBITAL TABLETS-CA1 is for use in dogs 6 months of age and older.
The safe use of PHENOBARBITAL TABLETS-CA1 in neonates and young dogs has not been established.
Careful monitoring of therapeutic, biochemical, and hematology parameters is important in dogs receiving PHENOBARBITAL TABLETS-CA1. Dogs receiving PHENOBARBITAL TABLETS-CA1 should also be carefully monitored when administering concurrent medications.
Dogs with decreased liver function may be predisposed to phenobarbital toxicosis.
Some dogs may experience epileptic episodes that are refractory or unresponsive to PHENOBARBITAL TABLETS-CA1 monotherapy.
Dogs should be monitored for adverse events when adjusting the dose to determine the optimal dose for each patient.
The safe use of PHENOBARBITAL TABLETS-CA1 has not been evaluated in dogs that are intended for breeding, or that are pregnant or lactating.
ADVERSE REACTIONS:
Based on a comprehensive review of publications evaluating phenobarbital, the following adverse reactions are associated with administration of phenobarbital:
General: ataxia, lethargy, increased water consumption, increased appetite, increased urination, hyperactivity, vomiting, diarrhea, and weight gain.
Hematologic: increased serum alkaline phosphatase (ALP), increased serum alanine transferase (ALT), increased serum gamma-glutamyl transferase (GGT), decreased serum total and free thyroxine (T4) concentrations, immune-mediated anemia, neutropenia, and thrombocytopenia. Rarely, idiosyncratic hepatotoxicity may also occur.
A retrospective study of data on 1083 cases from a poison control center included 268 dogs that ingested phenobarbital alone at a dose of ≤5 mg/kg. Among the 22 dogs with at least one adverse reaction, the following were the most common adverse reactions observed: drowsiness/lethargy, ataxia, agitated/irritable, vocalization, unable to rise, and vomiting. Each of these adverse reactions were reported in 2 or more dogs.
CONTACT INFORMATION:
For a copy of the Safety Data Sheet (SDS) or to report suspected adverse drug experiences, contact Genus Lifesciences Inc. at 1-833-599-7582. For additional adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae.
CLINICAL PHARMACOLOGY:
Mechanism of Action: Phenobarbital is a barbiturate thought to exert its antiepileptic activity by enhancing the action of gamma-aminobutyric acid (GABA) through binding to a site on the GABAA receptor/chloride channel. Barbiturates are also thought to decrease the influx of calcium into nerve cells and thereby decrease release of neurotransmitters.
Pharmacokinetics: The pharmacokinetics of phenobarbital in dogs have been described in published literature. Phenobarbital is considered a lipid-soluble drug exhibiting fast first-order absorption, high systemic availability, wide distribution, extensive hepatic metabolism, and slow and variable elimination from the body. Mean terminal elimination half-lives have been reported to range between 30 and 90 hours after oral administration. Half-lives tend to decrease after weeks of continuous administration. Steady state concentrations are often achieved after 2 or more weeks of daily or twice daily dosing. Phenobarbital has been reported to undergo self-induction of microsomal metabolism after weeks or months of daily administration, often requiring adjusting the dose after a certain period of time.
REASONABLE EXPECTATION OF EFFECTIVENESS:
A reasonable expectation of effectiveness may be demonstrated based on evidence such as, but not limited to, pilot data in the target species or studies from published literature.
PHENOBARBITAL TABLETS-CA1 is conditionally approved pending a full demonstration of effectiveness.
Additional information for Conditional Approvals can be found at www.fda.gov/animalca
A reasonable expectation of effectiveness for PHENOBARBITAL TABLETS-CA1 for the control of seizures associated with idiopathic epilepsy in dogs is supported by eight publications in the scientific literature: two clinical consensus statements, and four prospective and two retrospective published studies.
The American College of Veterinary Medicine (ACVIM) 2016 clinical consensus statement on seizure management in dogs states that phenobarbital as monotherapy is likely to be effective based on evaluation of masked, randomized clinical trials. The ACVIM consensus statement reviewed 8 studies for a total of 311 dogs where seizure reduction was evaluated following phenobarbital monotherapy. Over the 8 studies, 82% of the dogs (258/311 dogs) had >50% seizure reduction, including 31% (93/311) that were seizure-free, and 15% (48/311) did not improve. The consensus statement recommends that phenobarbital should be orally administered at a starting dose of 2.5 mg/kg twice daily with adjustments based on each dog's clinical response and tolerance to the drug. The article stated that the most effective and safe therapeutic range is 15-35 µg/mL (serum concentration), although effectiveness can be seen at lower concentrations.
The International Veterinary Epilepsy Task Force (IVETF) consensus statement for the medical treatment of canine epilepsy in Europe states that phenobarbital has a favorable pharmacokinetic profile, is relatively safe, and, when serum phenobarbital concentration is maintained within the therapeutic range of 25-35 µg/mL, is 60-93% effective in decreasing seizure frequency in dogs with idiopathic epilepsy. The IVETF recommended an oral starting dose of 2.5-3 mg/kg twice daily, tailoring the dose to the individual dog based on seizure control, adverse effects, and serum concentration monitoring.
Four prospective studies support the reasonable expectation of effectiveness of PHENOBARBITAL TABLETS-CA1 for the control of seizures associated with idiopathic epilepsy in dogs. In one prospective study 15 dogs were treated with phenobarbital for at least 6 months. Twelve dogs (80%) showed ≥50% reduction in seizures, including 6 dogs (40%) who had no seizures during the study. In a second study, 17 of 20 dogs treated with phenobarbital had no seizures during the study (85%), and 18 showed a decrease in seizure duration (88%). In a third study, 88 dogs were treated with phenobarbital for at least 6 months, resulting in ≥50% reduction in seizure frequency in 73 dogs (83%), including 51 dogs (58%) who had no seizures during the study. In a fourth study, 5 out of 6 dogs treated with phenobarbital (83.3%) showed >50% reduction in seizures.
The results of two retrospective studies also support the reasonable expectation of effectiveness of PHENOBARBITAL TABLETS-CA1 for the control of seizures associated with idiopathic epilepsy in dogs. In one study, 11 out of 37 (30%) phenobarbital-treated Labrador retrievers had no seizures during the study, and an additional 16 out of 37 (43%) dogs showed decreased seizure frequency, severity, and/or duration. In another study, 28 of 44 (64%) phenobarbital-treated dogs had a ≥50% reduction in seizure frequency including 9 dogs (20%) who had no seizures during the study.
TARGET ANIMAL SAFETY:
A weight of evidence approach was used to determine the safety of PHENOBARBITAL TABLETS-CA1. The evidence included a 1, 3, 5× margin of safety study, an analysis of reports of phenobarbital exposure reported to an animal poison control center, and a critical review of publicly available literature regarding the use of phenobarbital in dogs. Safety was bridged between PHENOBARBITAL TABLETS-CA1 and commercially available oral formulations of phenobarbital based upon published literature and formulation comparisons of qualitative, quantitative and dissolution data.
In the margin of safety study, PHENOBARBITAL TABLETS-CA1 was administered orally to dogs 1-2 years of age at 1, 3, and 5 times the maximum dose of 5 mg/kg every 12 hours. Beginning on the second day of dosing, 2 out of 8 dogs in the 3× group (15 mg/kg) and 7 out of 8 dogs in the 5× group (25 mg/kg) exhibited serious signs of toxicity, including significant sedation and ataxia. Due to the severity of the reactions, these seriously affected dogs were euthanized. The remaining dogs in the 3× and 5× groups had their doses reduced to 5 mg/kg. Due to the toxicity seen in the 3× and 5× groups the study was terminated early at 2 weeks.
In the retrospective study using an animal poison control center database, there were 1083 reports of dogs that ingested phenobarbital alone. Serious adverse events were reported in 50 of 1083 dogs (4.6%), and 43 of these 50 dogs received doses >25 mg/kg (>5× the highest recommended dose). The lowest dose associated with a serious adverse event was 14.29 mg/kg which represents a dose level of 2.86× the highest recommended dose. Serious adverse events included respiratory arrest, cardiac arrest, euthanasia, coma (unresponsive), and death.
In the systematic review of 30 scientific publications, administration of phenobarbital to dogs alone or in combination with other antiepileptic drugs resulted in adverse events including lethargy/sedation, ataxia, polyuria, polydipsia, polyphagia, weight gain, skin lesions (pruritus, alopecia, papules, pustules, erythema, and epidermal necrosis), changes in hematology parameters (neutropenia, anemia, thrombocytopenia), thyroid hormone changes (decreased thyroid hormone, increased TSH), and elevated liver enzymes including alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma glutamyl transferase (GGT), and glutamate dehydrogenase (GLDH). The liver enzyme elevations were generally attributed to enzyme induction rather than hepatotoxicity. Phenobarbital-induced hematological abnormalities appear to be unrelated to dose. Lymphadenopathy, tremors, hypoadrenocortical crisis, and pancreatitis were also reported. Adverse effects were generally seen either soon after the start of treatment or when serum levels of phenobarbital were high (>35 mcg/ml). Some adverse events appeared to resolve or improve with time or when there is a reduction in the phenobarbital dose. Hepatotoxicity appeared to be associated with higher dosages and chronic phenobarbital use.
HOW SUPPLIED:
PHENOBARBITAL TABLETS-CA1 are unflavored, bisected tablets containing 16.2, 32.4, 64.8, and 97.2 mg phenobarbital per tablet. PHENOBARBITAL TABLETS-CA1 are packaged in bottles of 100, 500 or 1000 tablets.
PRINCIPAL DISPLAY PANEL - 16.2 mg Tablet Bottle Label
PHENOBARBITAL
TABLETS-CA1
CIV
Antiepileptic for
oral use in dogs only.
16.2 mg
Conditionally approved by FDA pending a full demonstration
of effectiveness under application number 141-578.
It is a violation of federal law to use this product other
than as directed on the labeling.
Net Contents: 100 tablets
NDC: 51672-5322-1
TARO

PRINCIPAL DISPLAY PANEL - 32.4 mg Tablet Bottle Label
PHENOBARBITAL
TABLETS-CA1
CIV
Antiepileptic for
oral use in dogs only.
32.4 mg
Conditionally approved by FDA pending a full demonstration
of effectiveness under application number 141-578.
It is a violation of federal law to use this product other
than as directed on the labeling.
Net Contents: 100 tablets
NDC: 51672-5323-1
TARO

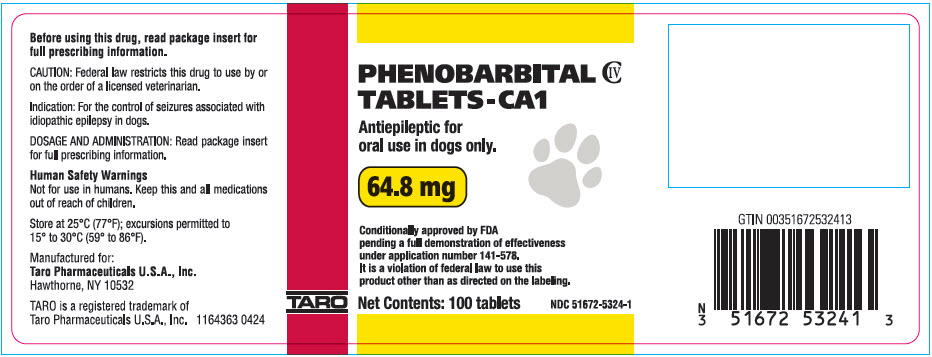
PRINCIPAL DISPLAY PANEL - 64.8 mg Tablet Bottle Label
PHENOBARBITAL
TABLETS-CA1
CIV
Antiepileptic for
oral use in dogs only.
64.8 mg
Conditionally approved by FDA
pending a full demonstration of effectiveness
under application number 141-578.
It is a violation of federal law to use this
product other than as directed on the labeling.
Net Contents: 100 tablets
NDC: 51672-5324-1
TARO
PRINCIPAL DISPLAY PANEL - 97.2 mg Tablet Bottle Label
PHENOBARBITAL
TABLETS-CA1
CIV
Antiepileptic for
oral use in dogs only.
97.2 mg
Conditionally approved by FDA
pending a full demonstration of effectiveness
under application number 141-578.
It is a violation of federal law to use this
product other than as directed on the labeling.
Net Contents: 100 tablets
NDC: 51672-5325-1
TARO

| PHENOBARBITAL
phenobarbital tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| PHENOBARBITAL
phenobarbital tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| PHENOBARBITAL
phenobarbital tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| PHENOBARBITAL
phenobarbital tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Sun Pharmaceutical Industries, Inc. (146974886) |
| Registrant - Genus Lifesciences Inc. (113290444) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Genus Lifesciences Inc. | 113290444 | MANUFACTURE, PACK, LABEL | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sunrise Pharmaceutical Inc. | 168522378 | PACK, LABEL | |