GOODSENSE TERBINAFINE HYDROCHLORIDE- terbinafine hydrochloride cream
GoodSense by
Drug Labeling and Warnings
GoodSense by is a Otc medication manufactured, distributed, or labeled by Geiss, Destin & Dunn, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
-
Directions
- adults and children 12 years and older
- use the tip of the cap to break the seal and open the tube
- wash the affected skin with soap and water and dry completely before applying
-
for athlete's foot wear well-fitting, ventilated shoes. Change shoes and socks at least once daily.
- between the toes only: apply twice a day (morning and night) for 1 week or as directed by a doctor.
- on the bottom or sides of the foot: apply twice a day (morning and night) for 2 weeks or as directed by a doctor.
- for jock itch and ringworm: apply once a day (morning or night) for 1 week or as directed by a doctor.
- wash hands after each use
- children under 12 years: ask a doctor
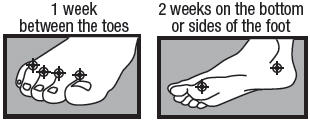
- adults and children 12 years and older
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 15 g Tube Carton
GOODSENSE®
NDC: 50804-080-01
Full Prescription Strength
Terbinafine Hydrochloride Cream 1%
Antifungal Cream
Compare to active ingredient in
Lamisil AT®*100%
SATISFACTION
GUARANTEEDNET WT 0.5 oz (15 g)

-
INGREDIENTS AND APPEARANCE
GOODSENSE TERBINAFINE HYDROCHLORIDE
terbinafine hydrochloride creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 50804-080 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Terbinafine Hydrochloride (UNII: 012C11ZU6G) (Terbinafine - UNII:G7RIW8S0XP) Terbinafine Hydrochloride 1 g in 100 g Inactive Ingredients Ingredient Name Strength benzyl alcohol (UNII: LKG8494WBH) cetyl alcohol (UNII: 936JST6JCN) cetyl palmitate (UNII: 5ZA2S6B08X) isopropyl myristate (UNII: 0RE8K4LNJS) polysorbate 60 (UNII: CAL22UVI4M) water (UNII: 059QF0KO0R) sodium hydroxide (UNII: 55X04QC32I) sorbitan monostearate (UNII: NVZ4I0H58X) stearyl alcohol (UNII: 2KR89I4H1Y) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50804-080-01 1 in 1 CARTON 1 15 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077511 07/02/2007 Labeler - Geiss, Destin & Dunn, Inc. (076059836)
Trademark Results [GoodSense]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 GOODSENSE 90845482 not registered Live/Pending |
L. Perrigo Company 2021-07-23 |
 GOODSENSE 85643777 4282338 Live/Registered |
BERRY GLOBAL FILMS, LLC 2012-06-05 |
 GOODSENSE 79097736 4184739 Live/Registered |
ZHANG JIN LAN 2011-04-11 |
 GOODSENSE 79097328 4181278 Live/Registered |
ZHANG JIN LAN 2011-04-21 |
 GOODSENSE 79067698 3761454 Live/Registered |
ZHEJIANG GOODSENSE FORKLIFT CO.,LTD. 2009-02-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.