Clobazam by Mylan Pharmaceuticals Inc. CLOBAZAM suspension
Clobazam by
Drug Labeling and Warnings
Clobazam by is a Prescription medication manufactured, distributed, or labeled by Mylan Pharmaceuticals Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CLOBAZAM ORAL SUSPENSION safely and effectively. See full prescribing information for CLOBAZAM ORAL SUSPENSION.
CLOBAZAM oral suspension, for oral use CIV
Initial U.S. Approval: 2011WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS
See full prescribing information for complete boxed warning.
Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death (5.1, 7.1).
- Reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate.
- Limit dosages and durations to the minimum required.
- Follow patients for signs and symptoms of respiratory depression and sedation.
INDICATIONS AND USAGE
Clobazam oral suspension is a benzodiazepine indicated for adjunctive treatment of seizures associated with Lennox-Gastaut syndrome (LGS) in patients 2 years of age or older (1)
DOSAGE AND ADMINISTRATION
- For doses above 5 mg/day administer in two divided doses (2.1)
- Patients ≤ 30 kg body weight: Initiate at 5 mg daily and titrate as tolerated up to 20 mg daily (2.1)
- Patients > 30 kg body weight: Initiate at 10 mg daily and titrate as tolerated up to 40 mg daily (2.1)
- Dosage adjustment needed in following groups:
- Reduce dose, or discontinue drug gradually (2.2)
- Measure prescribed amount of oral suspension using provided adapter and dosing syringe (2.3)
- Oral suspension: Can be taken with or without food (2.3)
DOSAGE FORMS AND STRENGTHS
- Oral Suspension: 2.5 mg/mL in 120 mL bottles (3)
CONTRAINDICATIONS
History of hypersensitivity to the drug or its ingredients (4)
WARNINGS AND PRECAUTIONS
- Somnolence or Sedation: Monitor for central nervous system (CNS) depression. Risk may be increased with concomitant use of other CNS depressants (5.2, 5.3)
- Withdrawal: Symptoms may occur with rapid dose reduction or discontinuation. Discontinue clobazam gradually (5.4)
- Serious Dermatological Reactions (including Stevens-Johnson syndrome and toxic epidermal necrolysis): Discontinue clobazam at first sign of rash unless the rash is clearly not drug-related (5.5)
- Physical and Psychological Dependence: Monitor patients with a history of substance abuse for signs of habituation and dependence (5.6, 9)
- Suicidal Behavior and Ideation: Monitor for suicidal thoughts or behaviors (5.7)
ADVERSE REACTIONS
Adverse reactions that occurred at least 10% more frequently than placebo in any clobazam dose included constipation, somnolence or sedation, pyrexia, lethargy, and drooling (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Mylan at 1-877-446-3679 (1-877-4-INFO-RX) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 6/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Information
2.2 Gradual Withdrawal
2.3 Important Administration Instructions
2.4 Dosage Adjustments in Geriatric Patients
2.5 Dosage Adjustments in CYP2C19 Poor Metabolizers
2.6 Patients with Renal Impairment
2.7 Dosage Adjustments in Patients with Hepatic Impairment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risks from Concomitant Use with Opioids
5.2 Potentiation of Sedation from Concomitant Use with Central Nervous System Depressants
5.3 Somnolence or Sedation
5.4 Withdrawal Symptoms
5.5 Serious Dermatological Reactions
5.6 Physical and Psychological Dependence
5.7 Suicidal Behavior and Ideation
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Opioids
7.2 CNS Depressants and Alcohol
7.3 Effect of Clobazam on Other Drugs
7.4 Effect of Other Drugs on Clobazam
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 CYP2C19 Poor Metabolizers
8.7 Renal Impairment
8.8 Hepatic Impairment
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
10 OVERDOSAGE
10.1 Signs and Symptoms of Overdosage
10.2 Management of Overdosage
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.5 Pharmacogenomics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS
Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death [see Warnings and Precautions (5.1), Drug Interactions (7.1)].
- Reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate.
- Limit dosages and durations to the minimum required.
- Follow patients for signs and symptoms of respiratory depression and sedation.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Information
A daily dose of clobazam oral suspension greater than 5 mg should be administered in divided doses twice daily; a 5 mg daily dose can be administered as a single dose. Dose patients according to body weight. Individualize dosing within each body weight group, based on clinical efficacy and tolerability. Each dose in Table 1 (e.g., 5 to 20 mg in ≤ 30 kg weight group) has been shown to be effective, although effectiveness increases with increasing dose [see Clinical Studies (14)].Do not proceed with dose escalation more rapidly than weekly, because serum concentrations of clobazam and its active metabolite require 5 and 9 days, respectively, to reach steady-state.
Table 1. Recommended Total Daily Dosing by Weight Group ≤ 30 kg Body Weight
> 30 kg Body Weight
Starting Dose
5 mg
10 mg
Starting Day 7
10 mg
20 mg
Starting Day 14
20 mg
40 mg
2.2 Gradual Withdrawal
As with all antiepileptic drugs and benzodiazepines, withdraw clobazam oral suspension gradually. Taper by decreasing the total daily dose by 5 to 10 mg/day on a weekly basis until discontinued [see Warnings and Precautions (5.4)].
2.3 Important Administration Instructions
Instruct patients to read the “Instructions for Use” carefully for complete directions on how to properly dose and administer clobazam oral suspension.
Clobazam Oral Suspension Oral Administration
Clobazam oral suspension can be taken with or without food [see Clinical Pharmacology (12.3)].
Shake clobazam oral suspension well before every administration. When administering the oral suspension, use only the oral dosing syringe provided with the product. Each carton includes two syringes, but only one syringe should be used for dosing. The second oral syringe is reserved as a replacement in case the first syringe is damaged or lost. Insert the provided adapter firmly into the neck of the bottle before first use and keep the adapter in place for the duration of the usage of the bottle. To withdraw the dose, insert the dosing syringe into the adapter and invert the bottle then slowly pull back the plunger to prescribed dose. After removing the syringe from the bottle adapter, slowly squirt clobazam oral suspension into the corner of the patient’s mouth. Replace the cap after each use. The cap fits over the adapter when the adapter is properly placed. See clobazam oral suspension “Instructions for Use” for complete instruction on how to properly dose and administer the clobazam oral suspension.
2.4 Dosage Adjustments in Geriatric Patients
Plasma concentrations at any given dose are generally higher in the elderly: proceed slowly with dose escalation. The starting dose should be 5 mg/day for all elderly patients. Then titrate elderly patients according to weight, but to half the dose presented in Table 1, as tolerated. If necessary and based upon clinical response, an additional titration to the maximum dose (20 mg/day or 40 mg/day, depending on weight) may be started on day 21 [see Use in Specific Populations (8.5)].
2.5 Dosage Adjustments in CYP2C19 Poor Metabolizers
In CYP2C19 poor metabolizers, levels of N-desmethylclobazam, clobazam’s active metabolite, will be increased. Therefore, in patients known to be CYP2C19 poor metabolizers, the starting dose should be 5 mg/day and dose titration should proceed slowly according to weight, but to half the dose presented in Table 1, as tolerated. If necessary and based upon clinical response, an additional titration to the maximum dose (20 mg/day or 40 mg/day, depending on the weight group) may be started on day 21 [see Use in Specific Populations (8.6), Clinical Pharmacology (12.5)].
2.6 Patients with Renal Impairment
No dose adjustment is required for patients with mild and moderate renal impairment. There is no experience with clobazam oral suspension in patients with severe renal impairment or end stage renal disease (ESRD). It is not known if clobazam or its active metabolite, N-desmethylclobazam, is dialyzable [see Use in Specific Populations (8.7), Clinical Pharmacology (12.3)].
2.7 Dosage Adjustments in Patients with Hepatic Impairment
Clobazam oral suspension is hepatically metabolized; however, there are limited data to characterize the effect of hepatic impairment on the pharmacokinetics of clobazam oral suspension. For this reason, proceed slowly with dosing escalations. For patients with mild to moderate hepatic impairment (Child-Pugh score 5 to 9), the starting dose should be 5 mg/day in both weight groups. Then titrate patients according to weight, but to half the dose presented in Table 1, as tolerated. If necessary and based upon clinical response, start an additional titration on day 21 to the maximum dose (20 mg/day or 40 mg/day, depending on the weight group). There is inadequate information about metabolism of clobazam oral suspension in patients with severe hepatic impairment. Therefore no dosing recommendation in those patients can be given [see Use in Specific Populations (8.8), Clinical Pharmacology (12.3)].
-
3 DOSAGE FORMS AND STRENGTHS
Clobazam Oral Suspension is a mixed berry flavored, white to off-white liquid suspension supplied in a bottle with child-resistant closure. The oral suspension is packaged with a dispenser set which contains two calibrated oral dosing syringes and a bottle adapter. Each bottle contains 120 mL of a white to off-white liquid suspension containing 2.5 mg/mL of clobazam for oral administration.
-
4 CONTRAINDICATIONS
Clobazam oral suspension is contraindicated in patients with a history of hypersensitivity to the drug or its ingredients. Hypersensitivity reactions have included serious dermatological reactions [see Warnings and Precautions (5.5)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Risks from Concomitant Use with Opioids
Concomitant use of benzodiazepines, including clobazam, and opioids may result in profound sedation, respiratory depression, coma, and death. Because of these risks, reserve concomitant prescribing of benzodiazepines and opioids for use in patients for whom alternative treatment options are inadequate.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioids alone. If a decision is made to prescribe clobazam concomitantly with opioids, prescribe the lowest effective dosages and minimum durations of concomitant use, and follow patients closely for signs and symptoms of respiratory depression and sedation. Advise both patients and caregivers about the risks of respiratory depression and sedation when clobazam is used with opioids [see Drug Interactions (7.1)].
5.2 Potentiation of Sedation from Concomitant Use with Central Nervous System Depressants
Since clobazam has a central nervous system (CNS) depressant effect, patients or their caregivers should be cautioned against simultaneous use with other CNS depressant drugs or alcohol, and cautioned that the effects of other CNS depressant drugs or alcohol may be potentiated [see Drug Interactions (7.2)].
5.3 Somnolence or Sedation
Clobazam causes somnolence and sedation. In clinical trials, somnolence or sedation was reported at all effective doses and was dose-related.
In general, somnolence and sedation begin within the first month of treatment and may diminish with continued treatment. Prescribers should monitor patients for somnolence and sedation, particularly with concomitant use of other central nervous system depressants. Prescribers should caution patients against engaging in hazardous activities requiring mental alertness, such as operating dangerous machinery or motor vehicles, until the effect of clobazam is known.
5.4 Withdrawal Symptoms
Abrupt discontinuation of clobazam should be avoided. Clobazam should be tapered by decreasing the dose every week by 5 to 10 mg/day until discontinuation [see Dosage and Administration (2.2)].
Withdrawal symptoms occurred following abrupt discontinuation of clobazam; the risk of withdrawal symptoms is greater with higher doses.
As with all antiepileptic drugs, clobazam should be withdrawn gradually to minimize the risk of precipitating seizures, seizure exacerbation, or status epilepticus.
Withdrawal symptoms (e.g., convulsions, psychosis, hallucinations, behavioral disorder, tremor, and anxiety) have been reported following abrupt discontinuance of benzodiazepines. The more severe withdrawal symptoms have usually been limited to patients who received excessive doses over an extended period of time, followed by an abrupt discontinuation. Generally milder withdrawal symptoms (e.g., dysphoria, anxiety, and insomnia) have been reported following abrupt discontinuance of benzodiazepines taken continuously at therapeutic doses for several months.
5.5 Serious Dermatological Reactions
Serious skin reactions, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), have been reported with clobazam in both children and adults during the postmarketing period. Patients should be closely monitored for signs or symptoms of SJS/TEN, especially during the first 8 weeks of treatment initiation or when re-introducing therapy. Clobazam should be discontinued at the first sign of rash, unless the rash is clearly not drug-related. If signs or symptoms suggest SJS/TEN, use of this drug should not be resumed and alternative therapy should be considered [see Contraindications (4)].
5.6 Physical and Psychological Dependence
Patients with a history of substance abuse should be under careful surveillance when receiving clobazam or other psychotropic agents because of the predisposition of such patients to habituation and dependence [see Drug Abuse and Dependence (9)].
5.7 Suicidal Behavior and Ideation
Antiepileptic drugs (AEDs), including clobazam, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted relative risk 1.8, 95% confidence interval [CI]: 1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence rate of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43%, compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug-treated patients in the trials and none in placebo-treated patients, but the number is too small to allow any conclusion about drug effect on suicide.
The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting drug treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed.
The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs used for any indication. The risk did not vary substantially by age (5 to 100 years) in the clinical trials analyzed. Table 2 shows absolute and relative risk by indication for all evaluated AEDs.
Table 2. Risk by Indication for Antiepileptic Drugs in the Pooled Analysis Indication
Placebo Patients with Events per 1,000 Patients
Drug Patients with Events per 1,000 Patients
Relative Risk:
Incidence of Drug Events in Drug Patients/Incidence in Placebo Patients
Risk Difference: Additional Drug Patients with Events per 1,000 Patients
Epilepsy
1.0
3.4
3.5
2.4
Psychiatric
5.7
8.5
1.5
2.9
Other
1.0
1.8
1.9
0.9
Total
2.4
4.3
1.8
1.9
The relative risk for suicidal thoughts or behavior was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.
Anyone considering prescribing clobazam or any other AED must balance the risk of suicidal thoughts or behavior with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
Patients, their caregivers, and families should be informed that AEDs increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of the signs and symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers.
-
6 ADVERSE REACTIONS
Clinically significant adverse reactions that appear in other sections of the labeling include the following:
- Risks from Concomitant Use with Opioids [see Warnings and Precautions (5.1)]
- Potentiation of Sedation from Concomitant Use with Central Nervous System Depressants [see Warnings and Precautions (5.2)]
- Somnolence or Sedation [see Warnings and Precautions (5.3)]
- Withdrawal Symptoms [see Warnings and Precautions (5.4)]
- Serious Dermatological Reactions [see Contraindications (4), Warnings and Precautions (5.5)]
- Physical and Psychological Dependence [see Warnings and Precautions (5.6)]
- Suicidal Behavior and Ideation [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
During its development for the adjunctive treatment of seizures associated with LGS, clobazam was administered to 333 healthy volunteers and 300 patients with a current or prior diagnosis of LGS, including 197 patients treated for 12 months or more. The conditions and duration of exposure varied greatly and included single- and multiple-dose clinical pharmacology studies in healthy volunteers and two double-blind studies in patients with LGS (Study 1 and 2) [see Clinical Studies (14)]. Only Study 1 included a placebo group, allowing comparison of adverse reaction rates on clobazam at several doses to placebo.
Adverse Reactions Leading to Discontinuation in an LGS Placebo Controlled Clinical Trial (Study 1)
The adverse reactions associated with clobazam treatment discontinuation in ≥ 1% of patients in decreasing order of frequency included lethargy, somnolence, ataxia, aggression, fatigue, and insomnia.
Most Common Adverse Reactions in an LGS Placebo Controlled Clinical Trial (Study 1)
Table 3 lists the adverse reactions that occurred in ≥ 5% of clobazam-treated patients (at any dose), and at a rate greater than placebo-treated patients, in the randomized, double-blind, placebo-controlled, parallel group clinical study of adjunctive AED therapy for 15 weeks (Study 1).
Table 3. Adverse Reactions Reported for ≥ 5% of Patients and More Frequently than Placebo in Any Treatment Group - * Maximum daily dose of 5 mg for ≤ 30 kg body weight; 10 mg for > 30 kg body weight
- † Maximum daily dose of 10 mg for ≤ 30 kg body weight; 20 mg for > 30 kg body weight
- ‡ Maximum daily dose of 20 mg for ≤ 30 kg body weight; 40 mg for > 30 kg body weight
Placebo
N = 59
%
Clobazam Dose Level
All Clobazam
N = 179
%
Low*
N = 58
%
Medium†
N = 62
%
High‡
N = 59
%
Gastrointestinal Disorders
Vomiting
5
9
5
7
7
Constipation
0
2
2
10
5
Dysphagia
0
0
0
5
2
General Disorders and Administration Site Conditions
Pyrexia
3
17
10
12
13
Irritability
5
3
11
5
7
Fatigue
2
5
5
3
5
Infections and Infestations
Upper respiratory tract infection
10
10
13
14
12
Pneumonia
2
3
3
7
4
Urinary tract infection
0
2
5
5
4
Bronchitis
0
2
0
5
2
Metabolism and Nutrition Disorders
Decreased appetite
3
3
0
7
3
Increased appetite
0
2
3
5
3
Nervous System Disorders
Somnolence or Sedation
15
17
27
32
26
Somnolence
12
16
24
25
22
Sedation
3
2
3
9
5
Lethargy
5
10
5
15
10
Drooling
3
0
13
14
9
Ataxia
3
3
2
10
5
Psychomotor hyperactivity
3
3
3
5
4
Dysarthria
0
2
2
5
3
Psychiatric Disorders
Aggression
5
3
8
14
8
Insomnia
2
2
5
7
5
Respiratory Disorders
Cough
0
3
5
7
5
6.2 Postmarketing Experience
These reactions are reported voluntarily from a population of uncertain size; therefore, it is not possible to estimate their frequency or establish a causal relationship to drug exposure. Adverse reactions are categorized by system organ class.
Blood Disorders: Anemia, eosinophilia, leukopenia, thrombocytopenia
Eye Disorders: Diplopia, vision blurred
Gastrointestinal Disorders: Abdominal distention
General Disorders and Administration Site Conditions: Hypothermia
Investigations: Hepatic enzyme increased
Musculoskeletal: Muscle spasms
Psychiatric Disorders: Agitation, anxiety, apathy, confusional state, depression, delirium, delusion, hallucination
Renal and Urinary Disorders: Urinary retention
Respiratory Disorders: Aspiration, respiratory depression
Skin and Subcutaneous Tissue Disorders: Rash, urticaria, angioedema, and facial and lip edema
-
7 DRUG INTERACTIONS
7.1 Opioids
The concomitant use of benzodiazepines and opioids increases the risk of respiratory depression because of actions at different receptor sites in the CNS that control respiration. Benzodiazepines interact at GABAA sites, and opioids interact primarily at mu receptors. When benzodiazepines and opioids are combined, the potential for benzodiazepines to significantly worsen opioid-related respiratory depression exists. Limit dosage and duration of concomitant use of benzodiazepines and opioids, and follow patients closely for respiratory depression and sedation [see Warnings and Precautions (5.1)].
7.2 CNS Depressants and Alcohol
Concomitant use of clobazam with other CNS depressants may increase the risk of sedation and somnolence [see Warnings and Precautions (5.2)].
Alcohol, as a CNS depressant, will interact with clobazam in a similar way and also increases clobazam’s maximum plasma exposure by approximately 50%. Therefore, caution patients or their caregivers against simultaneous use with other CNS depressant drugs or alcohol, and caution that the effects of other CNS depressant drugs or alcohol may be potentiated [see Warnings and Precautions (5.2)].
7.3 Effect of Clobazam on Other Drugs
Hormonal Contraceptives
Clobazam is a weak CYP3A4 inducer. As some hormonal contraceptives are metabolized by CYP3A4, their effectiveness may be diminished when given with clobazam. Additional non-hormonal forms of contraception are recommended when using clobazam [see Clinical Pharmacology (12.3), Patient Counseling Information (17)].
Drugs Metabolized by CYP2D6
Clobazam inhibits CYP2D6. Dose adjustment of drugs metabolized by CYP2D6 may be necessary [see Clinical Pharmacology (12.3)].
7.4 Effect of Other Drugs on Clobazam
Strong and Moderate Inhibitors of CYP2C19
Strong and moderate inhibitors of CYP2C19 may result in increased exposure to N-desmethylclobazam, the active metabolite of clobazam. This may increase the risk of dose-related adverse reactions. Dosage adjustment of clobazam may be necessary when co-administered with strong CYP2C19 inhibitors (e.g., fluconazole, fluvoxamine, ticlopidine) or moderate CYP2C19 inhibitors (e.g., omeprazole) [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to AEDs, such as clobazam, during pregnancy. Physicians are advised to recommend that pregnant patients taking clobazam enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry. This can be done by calling the toll-free number 1-888-233-2334, and must be done by patients themselves. Information on the registry can also be found at the website http://www.aedpregnancyregistry.org/.
Risk Summary
There are no adequate and well-controlled studies of clobazam in pregnant women. Available data suggest that the class of benzodiazepines is not associated with marked increases in risk for congenital anomalies. Although some early epidemiological studies suggested a relationship between benzodiazepine drug use in pregnancy and congenital anomalies such as cleft lip and or palate, these studies had considerable limitations. More recently completed studies of benzodiazepine use in pregnancy have not consistently documented elevated risks for specific congenital anomalies. There is insufficient evidence to assess the effect of benzodiazepine pregnancy exposure on neurodevelopment.
There are clinical considerations regarding exposure to benzodiazepines during the second and third trimester of pregnancy or immediately prior to or during childbirth. These risks include decreased fetal movement and/or fetal heart rate variability, “floppy infant syndrome,” dependence, and withdrawal [see Clinical Considerations and Human Data].
Administration of clobazam to pregnant rats and rabbits during the period of organogenesis or to rats throughout pregnancy and lactation resulted in developmental toxicity, including increased incidences of fetal malformations and mortality, at plasma exposures for clobazam and its major active metabolite, N-desmethylclobazam, below those expected at therapeutic doses in patients [see Animal Data]. Data for other benzodiazepines suggest the possibility of long-term effects on neurobehavioral and immunological function in animals following prenatal exposure to benzodiazepines at clinically relevant doses. Clobazam should be used during pregnancy only if the potential benefit to the mother justifies the potential risk to the fetus. Advise a pregnant woman and women of childbearing age of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively. The background risk of major birth defects and miscarriage for the indicated population is unknown.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Infants born to mothers who have taken benzodiazepines during the later stages of pregnancy can develop dependence, and subsequently withdrawal, during the postnatal period. Clinical manifestations of withdrawal or neonatal abstinence syndrome may include hypertonia, hyperreflexia, hypoventilation, irritability, tremors, diarrhea, and vomiting. These complications can appear shortly after delivery to 3 weeks after birth and persist from hours to several months depending on the degree of dependence and the pharmacokinetic profile of the benzodiazepine. Symptoms may be mild and transient or severe. Standard management for neonatal withdrawal syndrome has not yet been defined. Observe newborns who are exposed to clobazam in utero during the later stages of pregnancy for symptoms of withdrawal and manage accordingly.
Labor and Delivery
Administration of benzodiazepines immediately prior to or during childbirth can result in a floppy infant syndrome, which is characterized by lethargy, hypothermia, hypotonia, respiratory depression, and difficulty feeding. Floppy infant syndrome occurs mainly within the first hours after birth and may last up to 14 days. Observe exposed newborns for these symptoms and manage accordingly.
Data
Human Data
Congenital Anomalies
Although there are no adequate and well controlled studies of clobazam in pregnant women, there is information about benzodiazepines as a class. Dolovich et al. published a meta-analysis of 23 studies that examined the effects of benzodiazepine exposure during the first trimester of pregnancy. Eleven of the 23 studies included in the meta-analysis considered the use of chlordiazepoxide and diazepam and not other benzodiazepines. The authors considered case-control and cohort studies separately. The data from the cohort studies did not suggest an increased risk for major malformations (OR 0.90; 95% CI 0.61—1.35) or for oral cleft (OR 1.19; 95% CI 0.34—4.15). The data from the case-control studies suggested an association between benzodiazepines and major malformations (OR 3.01, 95% CI 1.32—6.84) and oral cleft (OR 1.79; 95% CI 1.13— 2.82). The limitations of this meta-analysis included the small number of reports included in the analysis, and that most cases for analyses of both oral cleft and major malformations came from only three studies. A follow up to that meta-analysis included 3 new cohort studies that examined risk for major malformations and one study that considered cardiac malformations. The authors found no new studies with an outcome of oral clefts. After the addition of the new studies, the odds ratio for major malformations with first trimester exposure to benzodiazepines was 1.07 (95% CI 0.91—1.25).
Neonatal Withdrawal and Floppy Infant Syndrome
Neonatal withdrawal syndrome and symptoms suggestive of floppy infant syndrome associated with administration of clobazam during the later stages of pregnancy and peripartum period have been reported in the postmarketing experience. Findings in published scientific literature suggest that the major neonatal side effects of benzodiazepines include sedation and dependence with withdrawal signs. Data from observational studies suggest that fetal exposure to benzodiazepines is associated with the neonatal adverse events of hypotonia, respiratory problems, hypoventilation, low Apgar score, and neonatal withdrawal syndrome.
Animal Data
In a study in which clobazam (0, 150, 450, or 750 mg/kg/day) was orally administered to pregnant rats throughout the period of organogenesis, embryofetal mortality and incidences of fetal skeletal variations were increased at all doses. The low-effect dose for embryofetal developmental toxicity in rats (150 mg/kg/day) was associated with plasma exposures (AUC) for clobazam and its major active metabolite, N-desmethylclobazam, lower than those in humans at the maximum recommended human dose (MRHD) of 40 mg/day.
Oral administration of clobazam (0, 10, 30, or 75 mg/kg/day) to pregnant rabbits throughout the period of organogenesis resulted in decreased fetal body weights, and increased incidences of fetal malformations (visceral and skeletal) at the mid and high doses, and an increase in embryofetal mortality at the high dose. Incidences of fetal variations were increased at all doses. The highest dose tested was associated with maternal toxicity (ataxia and decreased activity). The low-effect dose for embryofetal developmental toxicity in rabbits (10 mg/kg/day) was associated with plasma exposures for clobazam and N-desmethylclobazam lower than those in humans at the MRHD.
Oral administration of clobazam (0, 50, 350, or 750 mg/kg/day) to rats throughout pregnancy and lactation resulted in increased embryofetal mortality at the high dose, decreased pup survival at the mid and high doses and alterations in offspring behavior (locomotor activity) at all doses. The low-effect dose for adverse effects on pre- and postnatal development in rats (50 mg/kg/day) was associated with plasma exposures for clobazam and N-desmethylclobazam lower than those in humans at the MRHD.
8.2 Lactation
Risk Summary
Clobazam is excreted in human milk. Postmarketing experience suggests that breastfed infants of mothers taking benzodiazepines, such as clobazam, may have effects of lethargy, somnolence and poor sucking. The effect of clobazam on milk production is unknown. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for clobazam and any potential adverse effects on the breastfed infant from clobazam or from the underlying maternal condition. If exposing a breastfed infant to clobazam, observe for any potential adverse effects.
8.3 Females and Males of Reproductive Potential
Administration of clobazam to rats prior to and during mating and early gestation resulted in adverse effects on fertility and early embryonic development at plasma exposures for clobazam and its major active metabolite, N-desmethylclobazam, below those in humans at the MRHD [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
Safety and effectiveness in patients less than 2 years of age have not been established.
In a study in which clobazam (0, 4, 36, or 120 mg/kg/day) was orally administered to rats during the juvenile period of development (postnatal days 14 to 48), adverse effects on growth (decreased bone density and bone length) and behavior (altered motor activity and auditory startle response; learning deficit) were observed at the high dose. The effect on bone density, but not on behavior, was reversible when drug was discontinued. The no-effect level for juvenile toxicity (36 mg/kg/day) was associated with plasma exposures (AUC) to clobazam and its major active metabolite, N-desmethylclobazam, less than those expected at therapeutic doses in pediatric patients.
8.5 Geriatric Use
Clinical studies of clobazam did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. However, elderly subjects appear to eliminate clobazam more slowly than younger subjects based on population pharmacokinetic analysis. For these reasons, the initial dose in elderly patients should be 5 mg/day. Patients should be titrated initially to 10 to 20 mg/day.
Patients may be titrated further to a maximum daily dose of 40 mg if tolerated [see Dosage and Administration (2.4), Clinical Pharmacology (12.3)].
8.6 CYP2C19 Poor Metabolizers
Concentrations of clobazam’s active metabolite, N-desmethylclobazam, are higher in CYP2C19 poor metabolizers than in extensive metabolizers. For this reason, dosage modification is recommended [see Dosage and Administration (2.5), Clinical Pharmacology (12.3)].
8.7 Renal Impairment
The pharmacokinetics of clobazam were evaluated in patients with mild and moderate renal impairment. There were no significant differences in systemic exposure (AUC and Cmax) between patients with mild or moderate renal impairment and healthy subjects. No dose adjustment is required for patients with mild and moderate renal impairment. There is essentially no experience with clobazam in patients with severe renal impairment or ESRD. It is not known if clobazam or its active metabolite, N-desmethylclobazam, is dialyzable [see Dosage and Administration (2.6), Clinical Pharmacology (12.3)].
8.8 Hepatic Impairment
Clobazam is hepatically metabolized; however, there are limited data to characterize the effect of hepatic impairment on the pharmacokinetics of clobazam. For this reason, dosage adjustment is recommended in patients with mild to moderate hepatic impairment (Child-Pugh score 5 to 9). There is inadequate information about metabolism of clobazam in patients with severe hepatic impairment [see Dosage and Administration (2.7), Clinical Pharmacology (12.3)].
-
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
Clobazam oral suspension contains clobazam which is a Schedule IV controlled substance.
9.2 Abuse
Clobazam can be abused in a similar manner as other benzodiazepines, such as diazepam.
The pharmacological profile of clobazam is similar to that of other benzodiazepines listed in Schedule IV of the Controlled Substance Act, particularly in its potentiation of GABAergic transmission through its action on GABAA receptors, which leads to sedation and somnolence.
The World Health Organization epidemiology database contains reports of drug abuse, misuse, and overdoses associated with clobazam.
Drug abuse is the intentional non-therapeutic use of a drug, repeatedly or even sporadically, for its rewarding psychological or physiological effects.
9.3 Dependence
Dependence
Physical dependence is a state of adaptation that is manifested by a specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, decreasing blood levels of the drug, and/or administration of an antagonist. In clinical trials, cases of dependency were reported following abrupt discontinuation of clobazam.
The risk of dependence is present even with use of clobazam at the recommended dose range over periods of only a few weeks. The risk of dependence increases with increasing dose and duration of treatment. The risk of dependence is increased in patients with a history of alcohol or drug abuse.
Withdrawal
Abrupt discontinuation of clobazam causes withdrawal symptoms. As with other benzodiazepines, clobazam should be withdrawn gradually [see Dosage and Administration (2.2), Warnings and Precautions (5.4)].
In clobazam clinical pharmacology trials in healthy volunteers, the most common withdrawal symptoms after abrupt discontinuation were headache, tremor, insomnia, anxiety, irritability, drug withdrawal syndrome, palpitations, and diarrhea [see Warnings and Precautions (5.4)].
Other withdrawal reactions to clobazam reported in the literature include restlessness, panic attacks, profuse sweating, difficulty in concentrating, nausea and dry retching, weight loss, blurred vision, photophobia, and muscle pain and stiffness. In general, benzodiazepine withdrawal may cause seizures, psychosis, and hallucinations [see Warnings and Precautions (5.4)].
-
10 OVERDOSAGE
10.1 Signs and Symptoms of Overdosage
Overdose and intoxication with benzodiazepines, including clobazam, may lead to CNS depression, associated with drowsiness, confusion and lethargy, possibly progressing to ataxia, respiratory depression, hypotension, and, rarely, coma or death. The risk of a fatal outcome is increased in cases of combined poisoning with other CNS depressants, including opioids and alcohol.
10.2 Management of Overdosage
The management of clobazam overdose may include gastric lavage and/or administration of activated charcoal, intravenous fluid replenishment, early control of airway and general supportive measures, in addition to monitoring level of consciousness and vital signs. Hypotension can be treated by replenishment with plasma substitutes and, if necessary, with sympathomimetic agents.
The efficacy of supplementary administration of physostigmine (a cholinergic agent) or of flumazenil (a benzodiazepine antagonist) in clobazam overdose has not been assessed. The administration of flumazenil in cases of benzodiazepine overdose can lead to withdrawal and adverse reactions. Its use in patients with epilepsy is typically not recommended.
-
11 DESCRIPTION
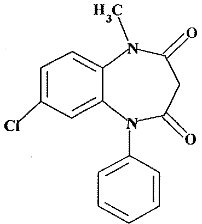
Clobazam is a white or almost white, crystalline powder with a slightly bitter taste; is slightly soluble in water, sparingly soluble in ethanol, and freely soluble in methylene chloride. The melting range of clobazam is from 183ºC to 184ºC. The molecular formula is C16H13ClN2O2 and the molecular weight is 300.7.
Clobazam is available for oral administration as a white to off-white liquid suspension containing clobazam at a concentration of 2.5 mg/mL. Inactive ingredients include citric acid monohydrate, dibasic sodium phosphate, magnesium aluminum silicate, maltitol solution, methylparaben, mixed berry flavor, nitrogen, polysorbate 80, propylene glycol, propylparaben, purified water, simethicone emulsion, sucralose and xanthan gum.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The exact mechanism of action for clobazam, a 1,5-benzodiazepine, is not fully understood but is thought to involve potentiation of GABAergic neurotransmission resulting from binding at the benzodiazepine site of the GABAA receptor.
12.2 Pharmacodynamics
Effects on Electrocardiogram
The effect of clobazam 20 mg and 80 mg administered twice daily on QTc interval was evaluated in a randomized, evaluator-blinded, placebo-, and active-controlled (moxifloxacin 400 mg) parallel thorough QT study in 280 healthy subjects. In a study with demonstrated ability to detect small effects, the upper bound of the one-sided 95% confidence interval for the largest placebo-adjusted, baseline-corrected QTc based on the Fridericia correction method was below 10 ms, the threshold for regulatory concern. Thus, at a dose two times the maximum recommended dose, clobazam did not prolong the QTc interval to any clinically relevant extent.
12.3 Pharmacokinetics
The peak plasma levels (Cmax) and the area under the curve (AUC) of clobazam are dose-proportional over the dose range of 10 to 80 mg following single- or multiple-dose administration of clobazam. Based on a population pharmacokinetic analysis, the pharmacokinetics of clobazam are linear from 5 to 160 mg/day. Clobazam is converted to N-desmethylclobazam which has about 1/5 the activity of clobazam. The estimated mean elimination half-lives (t½) of clobazam and N-desmethylclobazam were 36 to 42 hours and 71 to 82 hours, respectively.
Absorption
Clobazam is rapidly and extensively absorbed following oral administration. The time to peak concentrations (Tmax) of clobazam tablets under fasted conditions ranged from 0.5 to 4 hours after single- or multiple-dose administrations. The relative bioavailability of clobazam tablets compared to an oral solution is approximately 100%. After single dose administration of the oral suspension under fasted conditions, the Tmax ranged from 0.5 to 2 hours. Based on exposure (Cmax and AUC) of clobazam, clobazam tablets and oral suspension were shown to have similar bioavailability under fasted conditions. The administration of clobazam tablets with food or when crushed in applesauce does not affect absorption. Although not studied, the oral bioavailability of the oral suspension is unlikely to be affected under fed conditions.
Distribution
Clobazam is lipophilic and distributes rapidly throughout the body. The apparent volume of distribution at steady state was approximately 100 L. The in vitro plasma protein binding of clobazam and N-desmethylclobazam is approximately 80 to 90% and 70%, respectively.
Metabolism and Excretion
Clobazam is extensively metabolized in the liver, with approximately 2% of the dose recovered in urine and 1% in feces as unchanged drug. The major metabolic pathway of clobazam involves N-demethylation, primarily by CYP3A4 and to a lesser extent by CYP2C19 and CYP2B6. N-desmethylclobazam, an active metabolite, is the major circulating metabolite in humans, and at therapeutic doses, plasma concentrations are 3 to 5 times higher than those of the parent compound. Based on animal and in vitro receptor binding data, estimates of the relative potency of N-desmethylclobazam compared to parent compound range from 1/5 to equal potency. N-desmethylclobazam is extensively metabolized, mainly by CYP2C19. N-desmethylclobazam and its metabolites comprise ~94% of the total drug-related components in urine. Following a single oral dose of radiolabeled drug, approximately 11% of the dose was excreted in the feces and approximately 82% was excreted in the urine.
The polymorphic CYP2C19 is the major contributor to the metabolism of the pharmacologically active N-desmethylclobazam [see Clinical Pharmacology (12.5)]. In CYP2C19 poor metabolizers, levels of N-desmethylclobazam were 5-fold higher in plasma and 2- to 3-fold higher in the urine than in CYP2C19 extensive metabolizers.
Pharmacokinetics in Specific Populations
Age
Population pharmacokinetic analyses showed that the clearance of clobazam is lower in elderly subjects compared to other age groups (ages 2 to 64). Dosing should be adjusted in the elderly [see Dosage and Administration (2.4)].
Sex
Population pharmacokinetic analyses showed no difference in the clearance of clobazam between women and men.
Race
Population pharmacokinetic analyses including Caucasian (75%), African American (15%), and Asian (9%) subjects showed that there is no evidence of clinically significant effect of race on the clearance of clobazam.
Renal Impairment
The effect of renal impairment on the pharmacokinetics of clobazam was evaluated in patients with mild (creatinine clearance [CLCR] > 50 to 80 mL/min; N = 6) and moderate (CLCR = 30 to 50 mL/min; N = 6) renal dysfunction, with matching healthy controls (N = 6), following administration of multiple doses of clobazam 20 mg/day. There were insignificant changes in Cmax (3 to 24%) and AUC (≤ 13%) for clobazam or N-desmethylclobazam in patients with mild or moderate renal impairment compared to patients with normal renal function. Patients with severe renal impairment or ESRD were not included in this study.
Hepatic Impairment
There are limited data to characterize the effect of hepatic impairment on the pharmacokinetics of clobazam. In a small study, the pharmacokinetics of a 20 mg single oral dose of clobazam in 9 patients with liver impairment were compared to healthy controls (N = 6). The Cmax and the mean plasma clearance of clobazam, as well as the Cmax of N-desmethylclobazam, showed no significant change compared to the healthy controls. The AUC values of N-desmethylclobazam in these patients were not available. Adjust dosage in patients with hepatic impairment [see Dosage and Administration (2.7)].
Drug Interaction Studies
In Vitro Studies
Clobazam did not inhibit CYP1A2, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP3A4, UGT1A1, UGT1A4, UGT1A6, or UGT2B4 in vitro. N-desmethylclobazam showed weak inhibition of CYP2C9, UGT1A4, UGT1A6 and UGT2B4.
Clobazam and N-desmethylclobazam did not significantly increase CYP1A2 or CYP2C19 activities, but did induce CYP3A4 activity in a concentration-dependent manner. Clobazam and N-desmethylclobazam also increased UGT1A1 mRNA but at concentrations much higher than therapeutic levels. The potential for clobazam or N-desmethylclobazam to induce CYP2B6 and CYP2C8 has not been evaluated.
Clobazam and N-desmethylclobazam do not inhibit P-glycoprotein (P-gp), but are P-gp substrates.
In Vivo Studies
Potential for Clobazam to Affect Other Drugs
The effect of repeated 40 mg once-daily doses of clobazam on the pharmacokinetic profiles of single-dose dextromethorphan (CYP2D6 substrate), midazolam (CYP3A4 substrate), caffeine (CYP1A2 substrate), and tolbutamide (CYP2C9 substrate), was studied when these probe substrates were given as a drug cocktail (N = 18).
Clobazam increased AUC and Cmax of dextromethorphan by 90% and 59%, respectively, reflecting its inhibition of CYP2D6 in vivo. Drugs metabolized by CYP2D6 may require dose adjustment when used with clobazam.
Clobazam decreased the AUC and Cmax of midazolam by 27% and 24%, respectively, and increased the AUC and Cmax of the metabolite 1-hydroxymidazolam by 4-fold and 2-fold, respectively. This level of induction does not call for dosage adjustment of drugs that are primarily metabolized by CYP3A4 when used concomitantly with clobazam. Some hormonal contraceptives are metabolized by CYP3A4 and their effectiveness may be diminished when given with clobazam [see Drug Interactions (7.3)]. Repeated clobazam doses had no effect on caffeine and tolbutamide.
A population pharmacokinetic analysis indicated clobazam did not affect the exposure of valproic acid (a CYP2C9/2C19 substrate) or lamotrigine (a UGT substrate).
Potential for Other Drugs to Affect Clobazam
Co-administration of ketoconazole (a strong CYP3A4 inhibitor) 400 mg once-daily for 5 days increased clobazam AUC by 54%, with an insignificant effect on clobazam Cmax. There was no significant change in AUC and Cmax of N-desmethylclobazam (N = 18).
Strong (e.g., fluconazole, fluvoxamine, ticlopidine) and moderate (e.g., omeprazole) inhibitors of CYP2C19 may result in up to a 5-fold increase in exposure to N-desmethylclobazam, the active metabolite of clobazam, based on extrapolation from pharmacogenomic data [see Clinical Pharmacology (12.5)]. Dosage adjustment of clobazam may be necessary when co-administered with strong or moderate CYP2C19 inhibitors [see Drug Interactions (7.4)].
The effects of concomitant antiepileptic drugs that are CYP3A4 inducers (phenobarbital, phenytoin, and carbamazepine), CYP2C19 inducers (valproic acid, phenobarbital, phenytoin, and carbamazepine), and CYP2C19 inhibitors (felbamate and oxcarbazepine) were evaluated using data from clinical trials. Results of population pharmacokinetic analysis show that these concomitant antiepileptic drugs did not significantly alter the pharmacokinetics of clobazam or N-desmethylclobazam at steady-state.
Alcohol has been reported to increase the maximum plasma exposure of clobazam by approximately 50%. Alcohol may have additive CNS depressant effects when taken with clobazam [see Warnings and Precautions (5.2), Drug Interactions (7.2)].
12.5 Pharmacogenomics
The polymorphic CYP2C19 is the main enzyme that metabolizes the pharmacologically active N-desmethylclobazam. Compared to CYP2C19 extensive metabolizers, N-desmethylclobazam AUC and Cmax are approximately 3 to 5 times higher in poor metabolizers (e.g., subjects with *2/*2 genotype) and 2 times higher in intermediate metabolizers (e.g., subjects with *1/*2 genotype). The prevalence of CYP2C19 poor metabolism differs depending on racial/ethnic background. Dosage in patients who are known CYP2C19 poor metabolizers may need to be adjusted [see Dosage and Administration (2.5)].
The systemic exposure of clobazam is similar for both CYP2C19 poor and extensive metabolizers.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In mice, oral administration of clobazam (0, 6, 12, or 24 mg/kg/day) for 2 years did not result in an increase in tumors. The highest dose tested was approximately 3 times the maximum recommended human dose (MRHD) of 40 mg/day, based on body surface area (mg/m2).
In rats, oral administration of clobazam for 2 years resulted in increases in tumors of the thyroid gland (follicular cell adenoma and carcinoma) and liver (hepatocellular adenoma) at the mid and high doses. The low dose, not associated with an increase in tumors, was associated with plasma exposures (AUC) for clobazam and its major active metabolite, N-desmethylclobazam, less than that in humans at the MRHD.
Mutagenesis
Clobazam and the major active metabolite, N-desmethylclobazam, were negative for genotoxicity, based on data from a battery of in vitro (bacteria reverse mutation, mammalian clastogenicity) and in vivo (mouse micronucleus) assays.
Impairment of Fertility
In a fertility study in which clobazam (50, 350, or 750 mg/kg/day, corresponding to 12, 84 and 181 times the oral Maximum Recommended Human Dose, MRHD, of 40 mg/day based on mg/m2 body surface) was orally administered to male and female rats prior to and during mating and continuing in females to gestation day 6, increases in abnormal sperm and pre-implantation loss were observed at the highest dose tested. The no-effect level for fertility and early embryonic development in rats was associated with plasma exposures (AUC) for clobazam and its major active metabolite, N-desmethylclobazam, less than those in humans at the maximum recommended human dose of 40 mg/day.
-
14 CLINICAL STUDIES
The effectiveness of clobazam for the adjunctive treatment of seizures associated with Lennox-Gastaut syndrome was established in two multicenter controlled studies (Study 1 and Study 2). Both studies were similar in terms of disease characteristics and concomitant AED treatments. The most common concomitant AED treatments at baseline included: valproate, lamotrigine, levetiracetam, and topiramate.
Study 1: Study 1 (N = 238) was a randomized, double-blind, placebo-controlled study consisting of a 4-week baseline period followed by a 3-week titration period and 12-week maintenance period. Patients age 2 to 54 years with a current or prior diagnosis of LGS were stratified into 2 weight groups (12.5 kg to ≤ 30 kg or > 30 kg) and then randomized to placebo or one of three target maintenance doses of clobazam according to Table 5.
Table 5. Study 1 Total Daily Dose ≤ 30 kg Body Weight
> 30 kg Body Weight
Low Dose
5 mg daily
10 mg daily
Medium Dose
10 mg daily
20 mg daily
High Dose
20 mg daily
40 mg daily
Doses above 5 mg/day were administered in two divided doses.
The primary efficacy measure was the percent reduction in the weekly frequency of drop seizures (atonic, tonic, or myoclonic), also known as drop attacks, from the 4-week baseline period to 12-week maintenance period.
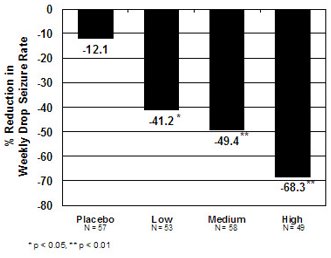
The pre-dosing baseline mean weekly drop seizure frequency was 98, 100, 61, and 105 for the placebo, low-, medium-, and high-dose groups, respectively. Figure 1 presents the mean percent reduction in weekly drop seizures from this baseline. All dose groups of clobazam were statistically superior (p ≤ 0.05) to the placebo group. This effect appeared to be dose dependent.
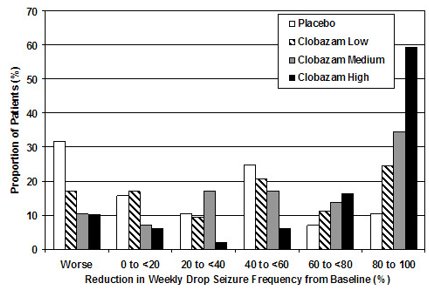
Figure 2 shows changes from baseline in weekly drop seizure frequency by category for patients treated with clobazam and placebo in Study 1. Patients in whom the seizure frequency increased are shown at left as “worse.” Patients in whom the seizure frequency decreased are shown in five categories.
There was no evidence that tolerance to the therapeutic effect of clobazam developed during the 3-month maintenance period.
Study 2: Study 2 (N = 68) was a randomized, double-blind comparison study of high- and low-dose clobazam, consisting of a 4-week baseline period followed by a 3-week titration period and 4-week maintenance period. Patients age 2 to 25 years with a current or prior diagnosis of LGS were stratified by weight, then randomized to either a low or high dose of clobazam, and then entered a 3-week titration period.
The primary efficacy measure was the percent reduction in the weekly frequency of drop seizures (atonic, tonic, or myoclonic), also known as drop attacks, from the 4-week baseline period to the 4-week maintenance period.
A statistically significantly greater reduction in seizure frequency was observed in the high-dose group compared to the low-dose group (median percent reduction of 93% vs 29%; p < 0.05).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Clobazam Oral Suspension is a mixed berry flavored, white to off-white liquid suspension supplied in a bottle with child-resistant closure. The oral suspension is packaged with a dispenser set which contains two calibrated oral dosing syringes and a bottle adapter.
Store and dispense clobazam oral suspension in its original bottle in an upright position. Use within 90 days of first opening the bottle, then discard any remainder.
NDC: 0378-8043-21
2.5 mg/mL supplied in a bottle containing 120 mL of liquid suspensionStore oral suspension at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
PHARMACIST: Dispense a Medication Guide and Instructions for Use with each prescription.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Risks from Concomitant Use with Opioids: Inform patients and caregivers that potentially fatal additive effects may occur if clobazam oral suspension is used with opioids and not to use such drugs concomitantly unless supervised by a healthcare provider [see Warnings and Precautions (5.1), Drug Interactions (7.1)].
Somnolence or Sedation: Advise patients or caregivers to check with their healthcare provider before clobazam oral suspension is taken with other CNS depressants such as other benzodiazepines, opioids, tricyclic antidepressants, sedating antihistamines, or alcohol [see Warnings and Precautions (5.2, 5.3)].
If applicable, caution patients about operating hazardous machinery, including automobiles, until they are reasonably certain that clobazam oral suspension does not affect them adversely (e.g., impair judgment, thinking or motor skills).
Increasing or Decreasing the Clobazam Oral Suspension Dose: Inform patients or caregivers to consult their healthcare provider before increasing the clobazam oral suspension dose or abruptly discontinuing clobazam oral suspension. Advise patients or caregivers that abrupt withdrawal of AEDs may increase their risk of seizure [see Dosage and Administration (2.2), Warnings and Precautions (5.4)].
Hypersensitivity: Inform patients or caregivers that clobazam oral suspension is contraindicated in patients with a history of hypersensitivity to the drug or its ingredients [see Warnings and Precautions (5.5)].
Interactions with Hormonal Contraceptives: Counsel women to also use non-hormonal methods of contraception when clobazam oral suspension is used with hormonal contraceptives and to continue these alternative methods for 28 days after discontinuing clobazam oral suspension to ensure contraceptive reliability [see Drug Interactions (7.3), Clinical Pharmacology (12.3)].
Serious Dermatological Reactions: Advise patients or caregivers that serious skin reactions have been reported in patients taking clobazam oral suspension. Serious skin reactions, including SJS/TEN, may need to be treated in a hospital and may be life-threatening. If a skin reaction occurs while taking clobazam oral suspension, patients or caregivers should consult with healthcare providers immediately [see Warnings and Precautions (5.5)].
Suicidal Thinking and Behavior: Counsel patients, their caregivers, and their families that AEDs, including clobazam oral suspension, may increase the risk of suicidal thoughts and behavior and advise them of the need to be alert for the emergence or worsening of symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts of self-harm. Patients should report behaviors of concern immediately to healthcare providers [see Warnings and Precautions (5.7)].
Pregnancy: Advise pregnant women and women of childbearing potential that the use of clobazam during pregnancy can cause fetal harm which may occur early in pregnancy before many women know they are pregnant. Instruct patients to notify their healthcare provider if they become pregnant or intend to become pregnant during therapy.When appropriate, prescribers should counsel pregnant women and women of childbearing potential about alternative therapeutic options.
Advise patients that there is a pregnancy exposure registry that collects information about the safety of antiepileptic drugs during pregnancy [see Use in Specific Populations (8.1)].
Nursing: Counsel patients that clobazam is excreted in breast milk. Instruct patients to notify their physician if they are breast feeding or intend to breast feed during therapy and counsel nursing mothers to observe their infants for poor sucking and somnolence [see Use in Specific Populations (8.2)].
-
Medication Guide
Clobazam Oral Suspension CIV
(kloe' ba zam)What is the most important information I should know about clobazam oral suspension?
- Do not stop taking clobazam oral suspension without first talking to your healthcare provider. Stopping clobazam oral suspension suddenly can cause serious side effects.
- Clobazam oral suspension is a benzodiazepine medicine. Benzodiazepines can cause severe drowsiness, breathing problems (respiratory depression), coma, and death when taken with opioid medicines.
-
Clobazam oral suspension can make you sleepy or dizzy and can slow your thinking and motor skills. This may get better over time.
- o Do not drive, operate heavy machinery, or do other dangerous activities until you know how clobazam oral suspension affects you.
- o Clobazam oral suspension may cause problems with your coordination, especially when you are walking or picking things up.
- Do not drink alcohol or take other drugs that may make you sleepy or dizzy while taking clobazam oral suspension until you talk to your healthcare provider. When taken with alcohol or drugs that cause sleepiness or dizziness, clobazam oral suspension may make your sleepiness or dizziness much worse.
-
Clobazam oral suspension can cause withdrawal symptoms.
- o Do not stop taking clobazam oral suspension all of a sudden without first talking to a healthcare provider. Stopping clobazam oral suspension suddenly can cause seizures that will not stop (status epilepticus), hearing or seeing things that are not there (hallucinations), shaking, nervousness, and stomach and muscle cramps.
- o Talk to your healthcare provider about slowly stopping clobazam oral suspension to avoid withdrawal symptoms.
-
Clobazam oral suspension can be abused and cause dependence.
- o Physical dependence is not the same as drug addiction. Your healthcare provider can tell you more about the differences between physical dependence and drug addiction.
- Clobazam oral suspension is a federal controlled substance (CIV) because it can be abused or lead to dependence. Keep clobazam oral suspension in a safe place to prevent misuse and abuse. Selling or giving away clobazam oral suspension may harm others, and is against the law. Tell your healthcare provider if you have ever abused or been dependent on alcohol, prescription medicines or street drugs.
-
Serious skin reactions have been seen when clobazam oral suspension is taken with other medicines and may require stopping its use. Do not stop taking clobazam oral suspension without first talking to your healthcare provider.
- o A serious skin reaction can happen at any time during your treatment with clobazam oral suspension, but is more likely to happen within the first 8 weeks of treatment. These skin reactions may need to be treated right away.
- o Call your healthcare provider immediately if you have skin blisters, rash, sores in the mouth, hives or any other allergic reaction.
- Like other antiepileptic drugs, clobazam oral suspension may cause suicidal thoughts or actions in a very small number of people, about 1 in 500.
Call your healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- new or worse anxiety
- trouble sleeping (insomnia)
- acting on dangerous impulses
- attempts to commit suicide
- feeling agitated or restless
- new or worse irritability
- an extreme increase in
- activity and talking (mania)
- new or worse depression
- panic attacks
- acting aggressive, being angry, or violent
- other unusual changes in behavior or mood
How can I watch for early symptoms of suicidal thoughts and actions?
- Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled.
Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes.
What is clobazam oral suspension?
Clobazam oral suspension is a prescription medicine used along with other medicines to treat seizures associated with Lennox-Gastaut syndrome in people 2 years of age or older.
It is not known if clobazam oral suspension is safe and effective in children less than 2 years old.
Do not take clobazam oral suspension if you:
- are allergic to clobazam or any of the ingredients in clobazam oral suspenison. See the end of this Medication Guide for a complete list of ingredients in clobazam oral suspension.
Before you take clobazam oral suspension, tell your healthcare provider about all your medical conditions, including if you:
- have liver or kidney problems
- have lung problems (respiratory disease)
- have or have had depression, mood problems, or suicidal thoughts or behavior
- use birth control medicine. Clobazam oral suspension may cause your birth control medicine to be less effective. Talk to your healthcare provider about the best birth control method to use.
-
are pregnant or plan to become pregnant. Clobazam oral suspension may harm your unborn baby.
- o Tell your healthcare provider right away if you become pregnant while taking clobazam oral suspension. You and your healthcare provider will decide if you should take clobazam oral suspension while you are pregnant.
- o Babies born to mothers receiving benzodiazepine medications (including clobazam oral suspension) late in pregnancy may be at some risk of experiencing breathing problems, feeding problems, dangerously low body temperature, and withdrawal symptoms.
- If you become pregnant while taking clobazam oral suspension, talk to your healthcare provider about registering with the North American Antiepileptic Drug Pregnancy Registry. You can register by calling 1-888-233-2334. For more information about the registry go to http://www.aedpregnancyregistry.org. The purpose of this registry is to collect information about the safety of antiepileptic drugs during pregnancy.
- Clobazam can pass into breast milk. Talk to your healthcare provider about the best way to feed your baby if you take clobazam oral suspension. You and your healthcare provider should decide if you will take clobazam oral suspension or breastfeed. You should not do both.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Taking clobazam oral suspension with certain other medicines can cause side effects or affect how well clobazam oral suspension or the other medicines work. Do not start or stop other medicines without talking to your healthcare provider.
How should I take clobazam oral suspension?
- Take clobazam oral suspension exactly as your healthcare provider tells you to take it.
- Your healthcare provider will tell you how much clobazam oral suspension to take and when to take it.
- Clobazam oral suspension can be taken with or without food.
- Shake the bottle of clobazam oral suspension right before you take each dose.
- Measure your dose of clobazam oral suspension using the bottle adapter and dosing syringes that come with your clobazam oral suspension.
- Read the Instructions for Use at the end of this Medication Guide for information on the right way to use clobazam oral suspension.
- Your healthcare provider may change your dose if needed. Do not change your dose of clobazam oral suspension without talking to your healthcare provider.
- Do not stop taking clobazam oral suspension without first talking to your healthcare provider.
- Stopping clobazam oral suspension suddenly can cause serious problems.
- If you take too much clobazam oral suspension, call your healthcare provider or go to the nearest hospital emergency room right away.
What should I avoid while taking clobazam oral suspension?
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how clobazam oral suspension affects you.
- Do not drink alcohol or take other medicines that may make you sleepy or dizzy while taking clobazam oral suspension until you talk to your healthcare provider. When taken with alcohol or medicines that cause sleepiness or dizziness, clobazam oral suspension may make your sleepiness or dizziness much worse.
What are the possible side effects of clobazam oral suspension?
Clobazam oral suspension may cause serious side effects, including: See “What is the most important information I should know about clobazam oral suspension?”
The most common side effects of clobazam oral suspension include:
- sleepiness
- cough
- acting aggressive, being angry, or violent
- tiredness
- drooling
- pain with urination
- difficulty sleeping
- problems with breathing
- constipation
- fever
- slurred speech
These are not all the possible side effects of clobazam oral suspension. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store clobazam oral suspension?
- Store clobazam oral suspension between 20° to 25°C (68° to 77°F).
Oral Suspension
- Replace the cap securely after opening.
- Store and dispense the oral suspension in its original bottle in an upright position. Use clobazam oral suspension within 90 days of first opening the bottle.
- After 90 days safely throw away any clobazam oral suspension that has not been used.
- Keep clobazam oral suspension and all medicines out of the reach of children.
General information about the safe and effective use of clobazam oral suspension.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use clobazam oral suspension for a condition for which it was not prescribed. Do not give clobazam oral suspension to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about clobazam oral suspension that is written for health professionals.What are the ingredients in clobazam oral suspension?
Oral Suspension
Active ingredient: clobazam
Inactive ingredients: citric acid monohydrate, dibasic sodium phosphate, magnesium aluminum silicate, maltitol solution, methylparaben, mixed berry flavor, nitrogen, polysorbate 80, propylene glycol, propylparaben, purified water, simethicone emulsion, sucralose and xanthan gum.
Manufactured for: Mylan Pharmaceuticals Inc., Morgantown, WV 26505 U.S.A.
For more information about clobazam oral suspension, call Mylan at 1-877-446-3679 (1-877-4-INFO-RX).
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Instructions for Use
Clobazam Oral Suspension CIV
(kloé ba zam)Read this Instructions for Use before using clobazam oral suspension and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your healthcare provider about your medical condition or treatment.
Prepare Clobazam Oral Suspension Dose
You will need the following supplies: See Figure A
- Clobazam oral suspension bottle
- Bottle adapter
- Oral dosing syringe (2 dosing oral syringes are included in the clobazam oral suspension box).
- Use only 1 oral syringe to take your dose of clobazam oral suspension. If you lose or damage the oral syringe, or cannot read the markings, use the other oral syringe.
Figure A
Step 1. Remove the clobazam oral suspension bottle, bottle adapter, and 1 oral syringe from the box.
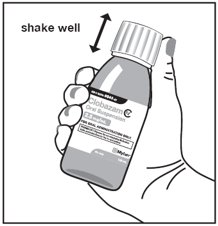
Step 2. Shake the bottle well before each use. See Figure B
Figure B
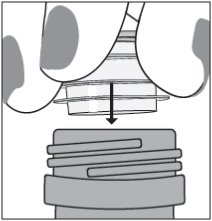
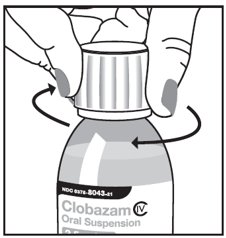
Step 3. Uncap the bottle and firmly insert the bottle adapter into the bottle until the adapter top is even with the bottle top. See Figure C
Figure C
Once the bottle adapter is in place, it should not be removed.
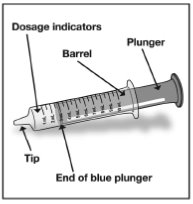
Step 4. Check your dose in milliliters (mL) as prescribed by your healthcare provider. Find this number on the oral syringe. Do not take more than the prescribed total dose in 1 day. See Figure D
Figure D
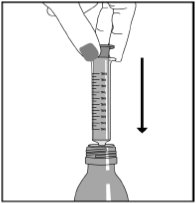
Step 5. Push the plunger all the way down and then insert the oral syringe into the upright bottle through the opening in the bottle adapter. See Figure E
Figure E
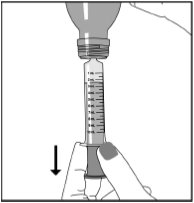
Step 6. With the oral syringe in place, turn the bottle upside down. Pull the plunger to the number of mLs needed (the amount of liquid medicine in Step 4). See Figure F
Figure F
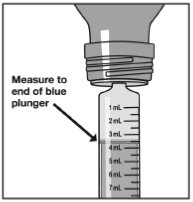
Measure the mLs of medicine using the end of the blue plunger. See Figure G
Figure G
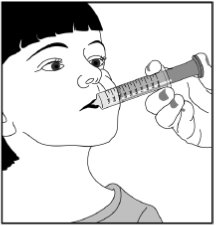
Step 7. Remove the oral syringe from the bottle adapter. Slowly squirt clobazam oral suspension directly into the corner of your mouth or your child’s mouth until all of the liquid medicine in the oral syringe is given. See Figure H
Figure H
Step 8. Cap the bottle tightly with the adapter in place. If the cap does not fit securely, check to see if the adapter is fully inserted. See Figure I
- Store and dispense clobazam oral suspension in its original bottle in an upright position at 20° to 25°C (68° to 77°F).
- Use clobazam oral suspension within 90 days of first opening bottle.
- After 90 days safely throw away any clobazam oral suspension that has not been used.
Figure I
Step 9. Wash the oral syringe after each use.
- To clean the oral syringe, take apart by removing the plunger completely. Pull plunger straight out of the barrel.
- The barrel and plunger can be washed with soap and water, rinsed, and allowed to dry.
- Do not wash the oral syringe in the dishwasher.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured for:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.Manufactured by:
DPT Laboratories, Ltd.
San Antonio, TX 78215 U.S.A.140975-0618
Revised: 6/2018
DPT:CLOBOS:R4 -
PRINCIPAL DISPLAY PANEL – 120 mL
NDC: 0378-8043-21
Clobazam
Oral Suspension CIV
2.5 mg/mLFOR ORAL
ADMINISTRATION ONLY.PHARMACIST: Dispense the accompanying
Medication Guide and Instructions for Use
to each patient.Rx only 120 mL
Store at 20° to 25°C (68° to
77°F). [See USP Controlled
Room Temperature.]STORE AND DISPENSE ORAL
SUSPENSION IN ORIGINAL
CONTAINER IN AN UPRIGHT
POSITION.Use within 90 days of first
opening, then discard any
remainder.Shake the bottle well before
each use.FOR ORAL ADMINISTRATION
ONLY.Keep this and all medication
out of the reach of children.This product is an oral
suspension liquid and is
supplied with two 10 mL oral
syringes for administration.Each mL contains:
Clobazam 2.5 mgUsual Dosage: See
accompanying prescribing
information.Manufactured for:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.DPT:8043:21:1C:R2
117508-0318
Mylan.com
-
INGREDIENTS AND APPEARANCE
CLOBAZAM
clobazam suspensionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0378-8043 Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLOBAZAM (UNII: 2MRO291B4U) (CLOBAZAM - UNII:2MRO291B4U) CLOBAZAM 2.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) MALTITOL (UNII: D65DG142WK) METHYLPARABEN (UNII: A2I8C7HI9T) NITROGEN (UNII: N762921K75) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color WHITE (white to off-white) Score Shape Size Flavor BERRY (mixed berry) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0378-8043-21 1 in 1 CARTON 11/07/2018 1 120 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211259 11/07/2018 Labeler - Mylan Pharmaceuticals Inc. (059295980)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.