SCOPOLAMINE patch, extended release
Scopolamine by
Drug Labeling and Warnings
Scopolamine by is a Prescription medication manufactured, distributed, or labeled by Ingenus Pharmaceuticals, LLC, RICONPHARMA LLC, Surmasis Pharmaceutical. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use SCOPOLAMINE TRANSDERMAL SYSTEM safely and effectively. See full prescribing information for SCOPOLAMINE TRANSDERMAL SYSTEM.
SCOPOLAMINE transdermal system
Initial U.S. Approval: 1979RECENT MAJOR CHANGES
Warnings and Precautions, Hyperthermia (5.5) 4/2025
INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
Application and Removal (2.1):
- Each scopolamine transdermal system delivers 1 mg of scopolamine over 3 days.
- Only wear one transdermal system at a time.
- Do not cut the transdermal system.
- Wash hands thoroughly with soap and water after application.
- Avoid touching or applying pressure to the transdermal system once applied.
- Upon removal, fold used transdermal system in half with sticky side together, discard to prevent accidental contact or ingestion, and wash the hands and application site with soap and water.
Recommended Dosage:
- Motion Sickness: Apply one transdermal system to the hairless area behind one ear at least 4 hours before antiemetic effect is required for use up to 3 days. If therapy for more than 3 days is required, remove the first transdermal system and apply a new transdermal system behind the other ear. (2.2)
- PONV: For surgeries other than cesarean section, apply one transdermal system behind the ear the evening before surgery and remove 24 hours following surgery. (2.2)
DOSAGE FORMS AND STRENGTHS
Transdermal system: 1 mg/3 days (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Acute Angle Closure Glaucoma: Monitor for increased intraocular pressure in patients with open-angle glaucoma and adjust glaucoma therapy as needed. Discontinue if signs or symptoms of acute angle closure glaucoma develop. (5.1)
- Neuropsychiatric Adverse Reactions: May cause psychiatric and cognitive effects, seizures and impair mental and/or physical abilities. Monitor patients for new or worsening psychiatric symptoms during treatment and during concomitant treatment with other drugs that are associated with similar psychiatric effects. (5.2, 7.1)
- Eclamptic Seizures in Pregnant Women: Avoid use in patients with severe preeclampsia. (5.3)
- Gastrointestinal and Urinary Disorders: Consider more frequent monitoring during treatment in patients suspected of having intestinal obstruction; patients with pyloric obstruction, patients with impeded urine flow or receiving other anticholinergic drugs. Discontinue if patient develops difficulty in urination. (5.4, 7.2)
- Hyperthermia: Serious adverse reactions have been reported postmarketing in adult and pediatric patients, including fatal cases. If symptoms occur, remove the transdermal system, and contact a healthcare provider. (5.5)
- Drug Withdrawal/Post-Removal Symptoms: Anticholinergic symptoms may occur 24 hours or more after removal of the transdermal system. (5.6)
- Blurred Vision: Avoid contact with the eyes. (2.1, 5.7)
- Magnetic Resonance Imaging (MRI) Skin Burns: Remove scopolamine transdermal system prior to MRI scan. (5.8)
ADVERSE REACTIONS
Most common adverse reactions are:
- Motion Sickness (>15%): dry mouth, drowsiness, blurred vision and dilation of the pupils. (6.1)
- PONV (≥ 3%): dry mouth, dizziness, somnolence, agitation, visual impairment, confusion, mydriasis and pharyngitis. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Ingenus Pharmaceuticals, LLC at 1-877-748-1970 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Drugs Causing Central Nervous System (CNS) Adverse Reactions: Monitor patients for CNS adverse reactions (drowsiness, dizziness or disorientations). (7.1)
- Anticholinergic Drugs: Consider more frequent monitoring during treatment in patients receiving other anticholinergic drugs. (7.2)
- Oral Drugs Absorbed in the Stomach: Monitor for increased or decreased therapeutic effect of the oral drug. (7.3)
- Interaction with Gastric Secretion Test: Discontinue use of scopolamine transdermal system 10 days prior to testing. (7.4)
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 5/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Application and Removal Instructions
2.2 Recommended Adult Dosage
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Acute Angle Closure Glaucoma
5.2 Neuropsychiatric Adverse Reactions
5.3 Eclamptic Seizures in Pregnant Women
5.4 Gastrointestinal and Urinary Disorders
5.5 Hyperthermia
5.6 Drug Withdrawal/Post-Removal Symptoms
5.7 Blurred Vision
5.8 Magnetic Resonance Imaging (MRI) Skin Burns
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Drugs Causing Central Nervous System (CNS) Adverse Reactions
7.2 Anticholinergic Drugs
7.3 Oral Drugs Absorbed in the Stomach
7.4 Interaction with Gastric Secretion Test
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal or Hepatic Impairment
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.3 Dependence
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Prevention of Motion Sickness
14.2 Prevention of Post-Operative Nausea and Vomiting
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Application and Removal Instructions
- Each scopolamine transdermal system is formulated to deliver in vivo approximately 1 mg of scopolamine over 3 days.
- Only wear one transdermal system at any time.
- Do not cut the transdermal system.
- Apply the transdermal system to the skin in the postauricular area (hairless area behind one ear).
- After the transdermal system is applied on the dry skin behind the ear, wash hands thoroughly with soap and water and dry hands [see Warnings and Precautions (5.7)].
- If the transdermal system becomes displaced, discard the transdermal system, and apply a new transdermal system on the hairless area behind the other ear.
- Once the transdermal system has been affixed, avoid touching or applying pressure to the transdermal system while it is being worn, since pressure exerted on it may cause scopolamine to ooze out at the edge.
- Upon removal, fold the used transdermal system in half with the sticky side together, and discard in household trash in a manner that prevents accidental contact or ingestion by children, pets or others.
- Wash the hands and application site with soap and water after transdermal system removal [see Warnings and Precautions (5.7)].
2.2 Recommended Adult Dosage
Motion Sickness
Apply one scopolamine transdermal system to the hairless area behind one ear at least 4 hours before the antiemetic effect is required – for use up to 3 days. If therapy is required for longer than 3 days, remove the first transdermal system and apply a new scopolamine transdermal system behind the other ear.
PONV
For surgeries other than cesarean section: Apply one scopolamine transdermal system the evening before scheduled surgery. Remove the transdermal system 24 hours following surgery.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Acute Angle Closure Glaucoma
The mydriatic effect of scopolamine may cause an increase in intraocular pressure resulting in acute angle closure glaucoma. Monitor intraocular pressure in patients with open angle glaucoma and adjust glaucoma therapy during scopolamine transdermal system use, as needed. Advise patients to immediately remove the transdermal system and contact their healthcare provider if they experience symptoms of acute angle closure glaucoma (e.g., eye pain or discomfort, blurred vision, visual halos or colored images in association with red eyes from conjunctival congestion and corneal edema).
5.2 Neuropsychiatric Adverse Reactions
Psychiatric Adverse Reactions
Scopolamine has been reported to exacerbate psychosis. Other psychiatric reactions have also been reported, including acute toxic psychosis, agitation, speech disorder, hallucinations, paranoia, and delusions [see Adverse Reactions (6.2)]. Monitor patients for new or worsening psychiatric symptoms during treatment with scopolamine transdermal system. Also, monitor patients for new or worsening psychiatric symptoms during concomitant treatment with other drugs that are associated with similar psychiatric effects [see Drug Interactions (7.1)]. In cases of psychiatric reactions occurring scopolamine transdermal system should be removed at once. If, despite this, the symptoms persist in a severe form, instruct patients to seek medical attention.
Seizures
Seizures and seizure-like activity have been reported in patients receiving scopolamine. Weigh this potential risk against the benefits before prescribing scopolamine transdermal system to patients with a history of seizures, including those receiving anti-epileptic medication or who have risk factors that can lower the seizure threshold.
Cognitive Adverse Reactions
Scopolamine can cause drowsiness, disorientation, and confusion. Discontinue scopolamine transdermal system if signs or symptoms of cognitive impairment develop. If, despite this, the symptoms persist in a severe form, instruct patients to seek medical attention. Elderly and pediatric patients may be more sensitive to the neurological and psychiatric effects of scopolamine transdermal system. Consider more frequent monitoring during treatment with scopolamine transdermal system in elderly patients [see Use in Specific Populations (8.5)]. Scopolamine transdermal system is not approved for use in pediatric patients [see Use in Specific Populations (8.4)].
Hazardous Activities
Scopolamine transdermal system may impair the mental and/or physical abilities required for the performance of hazardous tasks such as driving a motor vehicle, operating machinery or participating in underwater sports. Concomitant use of other drugs that cause central nervous system (CNS) adverse reactions (e.g., alcohol, sedatives, hypnotics, opiates, and anxiolytics) or have anticholinergic properties (e.g., other belladonna alkaloids, sedating antihistamines, meclizine, tricyclic antidepressants, and muscle relaxants) may increase this effect [see Drug Interactions (7.1)]. Inform patients not to operate motor vehicles or other dangerous machinery or participate in underwater sports until they are reasonably certain that scopolamine transdermal system does not affect them adversely.
5.3 Eclamptic Seizures in Pregnant Women
Eclamptic seizures have been reported in pregnant women with severe preeclampsia soon after injection of intravenous and intramuscular scopolamine [see Use in Specific Populations (8.1)]. Avoid use of scopolamine transdermal system in patients with severe preeclampsia.
5.4 Gastrointestinal and Urinary Disorders
Scopolamine, due to its anticholinergic properties, can decrease gastrointestinal motility and cause urinary retention. Consider more frequent monitoring during treatment with scopolamine transdermal system in patients suspected of having intestinal obstruction, patients with pyloric obstruction or patients with impeded flow of urine (e.g., in diseases of the prostate or urinary bladder neck obstruction) and patients receiving other anticholinergic drugs [see Drug Interactions (7.2)]. Discontinue scopolamine transdermal system in patients who develop difficulty in urination.
5.5 Hyperthermia
Serious adverse reactions of hyperthermia have been reported postmarketing in adult and pediatric patients receiving transdermal scopolamine, including fatal cases. Anticholinergic agents, including scopolamine, can increase core body temperature and reduce sweating, which may cause further increases in body temperature. Hyperthermia may be exacerbated by exposure to external heat sources or high environmental temperature. Pediatric and geriatric patients may be more susceptible to these anticholinergic effects on thermoregulation. Advise patients if body temperature increases, or they are not sweating in warm environmental conditions, to remove the transdermal system and contact their healthcare provider. Symptoms may persist following removal of the used transdermal system as there may be continued systemic absorption of scopolamine through the skin. Scopolamine transdermal system is not approved for use in pediatric patients [see Use in Specific Populations (8.4, 8.5)].
5.6 Drug Withdrawal/Post-Removal Symptoms
Discontinuation of scopolamine transdermal system, usually after several days of use, may result in withdrawal symptoms, such as disturbances of equilibrium, dizziness, nausea, vomiting, abdominal cramps, sweating, headache, mental confusion, muscle weakness, bradycardia and hypotension. The onset of these symptoms is generally 24 hours or more after the transdermal system has been removed. Instruct patients to seek medical attention if they experience severe symptoms.
5.7 Blurred Vision
Scopolamine can cause temporary dilation of the pupils resulting in blurred vision if it comes in contact with the eyes.
Advise patients to wash their hands thoroughly with soap and water and dry their hands immediately after handling the transdermal system, do not touch the system while wearing it, and wash hands and the application site with soap and water after transdermal system removal [see Dosage and Administration (2.1)].
5.8 Magnetic Resonance Imaging (MRI) Skin Burns
Scopolamine transdermal system contains an aluminized membrane. Skin burns have been reported at the application site in patients wearing an aluminized transdermal system during an MRI scan. Remove scopolamine transdermal system before undergoing an MRI.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in labeling:
- Acute Angle Closure Glaucoma [see Warnings and Precautions (5.1)]
- Neuropsychiatric Adverse Reactions [see Warnings and Precautions (5.2)]
- Eclamptic Seizures in Pregnant Women [see Warnings and Precautions (5.3)]
- Gastrointestinal and Urinary Disorders [see Warnings and Precautions (5.4)]
- Hyperthermia [see Warnings and Precautions (5.5)]
- Drug Withdrawal/Post-Removal Symptoms [see Warnings and Precautions (5.6)]
- Blurred Vision [see Warnings and Precautions (5.7)]
- MRI Skin Burns [see Warnings and Precautions (5.8)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Motion Sickness
The most common adverse reaction (approximately two thirds) was dry mouth. Less common adverse reactions, included drowsiness (less than one sixth), blurred vision and dilation of the pupils.
PONV
Common adverse reactions, occurring in at least 3% of patients in PONV clinical trials are shown in Table 1.
Table 1 Common Adverse Reactions* in Surgical Patients for the Prevention of PONV - * occurring in at least 3% of patients and at a rate higher than placebo
Scopolamine Transdermal System
% (N=461)
Placebo
% (N=457)
Dry mouth 29 16 Dizziness 12 7 Somnolence 8 4 Agitation 6 4 Visual Impairment 5 3 Confusion 4 3 Mydriasis 4 0 Pharyngitis 3 2 6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of scopolamine transdermal system. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Psychiatric disorders: acute psychosis including: disorientation, hallucinations, and paranoia
Nervous system disorders: amnesia, coordination abnormalities, disturbance in attention, headache, restlessness, speech disorder
General disorders and administration site conditions: application site reactions (including blistering, burning, pruritus, and rash) and hyperthermia
Eye disorders: amblyopia, angle closure glaucoma, dry eyes, eyelid irritation, eye pruritus
Skin and subcutaneous tissue disorders: erythema, rash generalized, skin irritation
Renal and urinary disorders: dysuria
Ear and labyrinth disorders: vertigo
-
7 DRUG INTERACTIONS
7.1 Drugs Causing Central Nervous System (CNS) Adverse Reactions
The concurrent use of scopolamine transdermal system with other drugs that cause CNS adverse reactions of drowsiness, dizziness or disorientation (e.g., sedatives, hypnotics, opiates, anxiolytics and alcohol) or have anticholinergic properties (e.g., other belladonna alkaloids, sedating antihistamines, meclizine, tricyclic antidepressants, and muscle relaxants) may potentiate the effects of scopolamine transdermal system [see Warnings and Precautions (5.2)]. Either scopolamine transdermal system or the interacting drug should be chosen, depending on the importance of the drug to the patient. If the interacting drug cannot be avoided, monitor patients for CNS adverse reactions.
7.2 Anticholinergic Drugs
Concomitant use of scopolamine with other drugs having anticholinergic properties may increase the risk of CNS adverse reactions [see Drug Interactions (7.1)], intestinal obstruction and/or urinary retention. Consider more frequent monitoring during treatment with scopolamine transdermal system in patients receiving anticholinergic drugs [see Warnings and Precautions (5.2, 5.4)].
7.3 Oral Drugs Absorbed in the Stomach
Scopolamine transdermal system, as an anticholinergic, may delay gastric and upper gastrointestinal motility and, therefore, the rate of absorption of other orally administered drugs. Monitor patients for modified therapeutic effect of concomitant orally administered drugs with a narrow therapeutic index.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Eclamptic seizures have been reported in pregnant women with severe preeclampsia soon after injection of intravenous or intramuscular scopolamine. Avoid use of scopolamine transdermal system in patients with severe preeclampsia [see Warnings and Precautions (5.3) and Data]. Available data from observational studies and postmarketing reports with scopolamine use in pregnant women have not identified a drug associated risk of major birth defects, miscarriage, or adverse fetal outcomes.
In animal studies, there was no evidence of adverse developmental effects with intravenous administration of scopolamine hydrobromide revealed in rats. Embryotoxicity was observed in rabbits at intravenous doses producing plasma levels approximately 100 times the levels achieved in humans using a transdermal system.
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Human Data
In published case reports, two pregnant patients with severe preeclampsia were administered intravenous and intramuscular scopolamine, respectively, and developed eclamptic seizures soon after scopolamine administration [see Warnings and Precautions (5.3)].
Animal Data
In animal reproduction studies, when pregnant rats and rabbits received scopolamine hydrobromide by daily intravenous injection, no adverse effects were observed in rats. An embryotoxic effect was observed in rabbits at doses producing plasma levels approximately 100 times the levels achieved in humans using a transdermal system. Scopolamine administered parenterally to rats and rabbits at doses higher than the dose delivered by scopolamine transdermal system did not affect uterine contractions or increase the duration of labor.
8.2 Lactation
Risk Summary
Scopolamine is present in human milk. There are no available data on the effects of scopolamine on the breastfed infant or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for scopolamine transdermal system and any potential adverse effects on the breastfed child from scopolamine transdermal system or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of scopolamine transdermal system have not been established in pediatric patients. Pediatric patients are particularly susceptible to the anticholinergic adverse reactions of scopolamine, including neurologic and psychiatric adverse reactions and drug withdrawal syndrome. Serious adverse reactions of acute psychosis (e.g., disorientation, hallucinations), amblyopia, hyperthermia (including a fatal case), and mydriasis have also been reported in pediatric patients [see Warnings and Precautions (5.2, 5.5)].
8.5 Geriatric Use
Clinical trials of scopolamine transdermal system did not include sufficient number of subjects aged 65 years and older to determine if they respond differently from younger subjects. In other clinical experience, elderly patients had an increased risk of neurologic and psychiatric adverse reactions, such as hallucinations, confusion, dizziness and drug withdrawal syndrome [see Warnings and Precautions (5.2, 5.6)]. Consider more frequent monitoring for CNS adverse reactions during treatment with scopolamine transdermal system in geriatric patients [see Warnings and Precautions (5.2)].
Serious adverse reactions of hyperthermia have been reported postmarketing in geriatric patients receiving transdermal scopolamine, including a fatal case. Geriatric patients may be more susceptible to the anticholinergic effects of disruption in thermoregulation. Advise patients if body temperature increases, or they are not sweating in warm environmental conditions, to remove the transdermal system and contact their healthcare provider [see Warnings and Precautions (5.5)].
8.6 Renal or Hepatic Impairment
Scopolamine transdermal system has not been studied in patients with renal or hepatic impairment. Consider more frequent monitoring during treatment with scopolamine transdermal system in patients with renal or hepatic impairment because of the increased risk of CNS adverse reactions [see Warnings and Precautions (5.2)].
-
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
Scopolamine transdermal system contains scopolamine, which is not a controlled substance.
9.3 Dependence
Termination of scopolamine transdermal system, usually after several days of use, may result in withdrawal symptoms such as disturbances of equilibrium, dizziness, nausea, vomiting, abdominal cramps, sweating, headache, mental confusion, muscle weakness, bradycardia and hypotension. These withdrawal symptoms indicate that scopolamine, like other anticholinergic drugs, may produce physical dependence. The onset of these symptoms, generally 24 hours or more after the transdermal system has been removed, can be severe and may require medical intervention [see Warnings and Precautions (5.6)].
-
10 OVERDOSAGE
Anticholinergic toxicity includes both central and peripheral signs and symptoms: agitation, central nervous system effects (e.g., coma, confusion, hallucinations, lethargy, seizures, somnolence), decreased bowel sounds, dry flushed skin, dry mouth, hyperthermia, hypertension, supraventricular arrhythmias, tachycardia, urinary retention, visual disturbances (e.g., amblyopia, mydriasis). These symptoms can be severe and may require medical intervention.
In cases of toxicity remove the scopolamine transdermal system. Serious symptomatic cases of overdosage involving multiple transdermal system applications and/or ingestion may be managed by initially ensuring the patient has an adequate airway and supporting respiration and circulation. This should be rapidly followed by removal of all transdermal systems from the skin and the mouth. If there is evidence of transdermal system ingestion, endoscopic removal of swallowed transdermal systems, or administration of activated charcoal should be considered, as indicated by the clinical situation. In any case where there is serious overdosage or signs of evolving acute toxicity, continuous monitoring of vital signs and ECG, establishment of intravenous access, and administration of oxygen are all recommended.
The signs and symptoms of overdose/toxicity due to scopolamine should be carefully distinguished from the occasionally observed syndrome of withdrawal [see Warnings and Precautions (5.6)]. Although mental confusion and dizziness may be observed with both acute toxicity and withdrawal, other characteristic findings differ: tachyarrhythmias, dry skin, and decreased bowel sounds suggest anticholinergic toxicity, while bradycardia, headache, nausea and abdominal cramps, and sweating suggest post-removal withdrawal.
If over-exposure occurs, call the Poison Help line at 1-800-222-1222 for current information on the management of poisoning or overdosage.
-
11 DESCRIPTION
Scopolamine transdermal system is designed for continuous release of scopolamine following application to an area of intact skin on the head, behind the ear. Each system contains 1.5 mg of scopolamine base. Scopolamine is (9-methyl-3-oxa-9-azatricyclo[3.3.1.02,4]nonan-7-yl) 3-hydroxy-2 phenylpropanoate. The empirical formula is C17H21NO4 and its structural formula is:
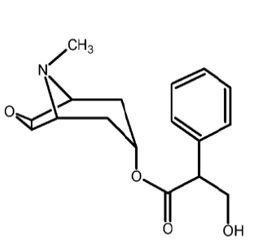
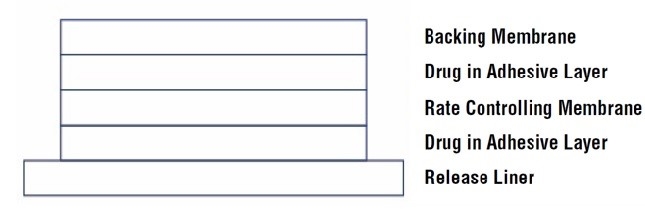
Scopolamine has a molecular weight of 303.35 and a pKa of 7.55-7.81. The scopolamine transdermal system is a circular, 0.2 mm thick, 2.5 cm2 film with four layers. Proceeding from the visible surface towards the surface attached to the skin, these layers are: (1) a backing membrane of tan-colored, aluminized, polyester film printed with brown ink; (2) a drug adhesive layer of scopolamine, acrylic adhesive and isopropyl myristate; (3) an ethylene vinyl acetate membrane that controls the rate of delivery of scopolamine from the system to the skin surface; and (4) a drug adhesive layer of scopolamine, acrylic adhesive and isopropyl myristate. A release liner of siliconized polyester, which covers the adhesive layer, is removed before the system is used. The brown imprinting ink contains FD&C yellow no. 6 aluminum lake, polyamide resin, polytetrafluoroethylene, polyethylene wax, carbon black and quinacridone red pigment.
Cross section of the system:
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Scopolamine, a belladonna alkaloid, is an anticholinergic. Scopolamine acts: i) as a competitive inhibitor at postganglionic muscarinic receptor sites of the parasympathetic nervous system, and ii) on smooth muscles that respond to acetylcholine but lack cholinergic innervation. It has been suggested that scopolamine acts in the central nervous system (CNS) by blocking cholinergic transmission from the vestibular nuclei to higher centers in the CNS and from the reticular formation to the vomiting center. Scopolamine can inhibit the secretion of saliva and sweat, decrease gastrointestinal secretions and motility, cause drowsiness, dilate the pupils, increase heart rate, and depress motor function.
12.3 Pharmacokinetics
The system is formulated to deliver approximately 1 mg of scopolamine to the systemic circulation over 3 days.
Absorption
Following application to the skin behind the ear, circulating plasma concentrations are detected within 4 hours with peak concentrations being obtained, on average, within 24 hours. The average plasma concentration produced is 87 pg/mL (0.28 nM) for free scopolamine and 354 pg/mL for total scopolamine (free + conjugates). Following removal of the used transdermal system, there is some degree of continued systemic absorption of scopolamine bound in the skin layers.
Distribution
The distribution of scopolamine is not well characterized. It crosses the placenta and the blood brain barrier and may be reversibly bound to plasma proteins.
Elimination
Metabolism and Excretion
Scopolamine is metabolized and conjugated with less than 5% of the total dose appearing unchanged in the urine. The enzymes responsible for metabolizing scopolamine are unknown. The exact elimination pattern of scopolamine has not been determined. Following transdermal system removal, plasma concentrations of scopolamine decline in a log linear fashion with an observed half-life of 9.5 hours. Less than 10% of the total dose is excreted in the urine as the parent drug and metabolites over 108 hours.
Drug Interaction Studies
An in vitro study using human hepatocytes examined the induction of CYP1A2 and CYP3A4 by scopolamine. Scopolamine did not induce CYP1A2 and CYP3A4 isoenzymes at the concentrations up to 10 nM. In an in vitro study using human liver microsomes which evaluated the inhibition of CYP1A2, 2C8, 2C9, 2C19, 2D6 and 3A4, scopolamine did not inhibit these cytochrome P450 isoenzymes at the concentrations up to 1 micromolar. No in vivo drug-drug interaction studies have been conducted.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term studies in animals have been conducted to evaluate the carcinogenic potential of scopolamine. The mutagenic potential of scopolamine has not been evaluated.
Fertility studies were performed in female rats and revealed no evidence of impaired fertility or harm to the fetus due to scopolamine hydrobromide administered by daily subcutaneous injection. Maternal body weights were reduced in the highest-dose group (plasma level approximately 500 times the level achieved in humans using a transdermal system). However, fertility studies in male animals were not performed.
-
14 CLINICAL STUDIES
14.1 Prevention of Motion Sickness
In 195 adult subjects of different racial origins who participated in clinical efficacy studies at sea or in a controlled motion environment, there was a 75% reduction in the incidence of motion-induced nausea and vomiting. Scopolamine transdermal system was applied from 4 to 16 hours prior to the onset of motion in these studies.
14.2 Prevention of Post-Operative Nausea and Vomiting
A clinical efficacy study evaluated 168 adult female patients undergoing gynecological surgery with anesthesia and opiate analgesia. Patients received scopolamine transdermal system or placebo applied approximately 11 hours before anesthesia/opiate analgesia. No retching/vomiting during the 24-hour post-operative period was reported in 79% of those treated with scopolamine transdermal system compared to 72% of those receiving placebo. When the need for additional antiemetic medication was assessed during the same period, there was no need for medication in 89% of patients treated with scopolamine transdermal system as compared to 72% of placebo-treated patients.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Scopolamine transdermal system 1 mg/3 days is available as the following:
Carton of 4 transdermal systems, packaged into individual foil pouches. NDC: 50742-505-04
Carton of 10 transdermal systems, packaged into individual foil pouches. NDC: 50742-505-10
Carton of 24 transdermal systems, packaged into individual foil pouches. NDC: 50742-505-24
Store at controlled room temperature between 68°F to 77°F (20°C to 25°C).
Store pouch(es) in an upright position.
Do not bend or roll pouch(es).
Wash hands thoroughly with soap and water immediately after handling the transdermal system. Avoid touching the system during the treatment. Upon removal, fold the used transdermal system in half with the sticky side together, and discard in household trash in a manner that prevents accidental contact or ingestion by children, pets or others. Wash the hands and application site with soap and water after transdermal system removal [see Dosage and Administration (2.1), Warnings and Precautions (5.7)].
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Administration Instructions
Counsel patients on how to apply and remove the transdermal system [see Dosage and Administration (2.1)]:
- Only wear one transdermal system at any time.
- Do not cut the transdermal system.
- Apply the transdermal system to the skin in the postauricular (hairless area behind one ear) area.
- After the transdermal system is applied on the dry skin behind the ear, wash hands thoroughly with soap and water and dry hands.
- If the transdermal system becomes displaced, discard the transdermal system, and apply a new transdermal system on the hairless area behind the other ear.
- Once the transdermal system has been affixed, avoid touching or applying pressure to the transdermal system, since pressure exerted on it may cause scopolamine to ooze out at the edge.
- Upon removal, fold the used transdermal system in half with the sticky side together, and discard in household trash in a manner that prevents accidental contact or ingestion by children, pets or others.
- Wash the hands and application site with soap and water after transdermal system removal.
Patients with Open-Angle Glaucoma
Advise patients with open-angle glaucoma to remove the scopolamine transdermal system immediately and contact their healthcare provider if they experience symptoms of acute angle closure glaucoma, including pain and reddening of the eyes, accompanied by dilated pupils, blurred vision and/or seeing halos around lights [see Warnings and Precautions (5.1)].
Neuropsychiatric Adverse Reactions
- Advise patients that psychiatric adverse reactions may occur, especially in patients with a past psychiatric history or in those receiving other drugs also associated with psychiatric effects, and to report to their healthcare provider any new or worsening psychiatric symptoms.
- Advise patients to discontinue scopolamine transdermal system and contact a healthcare provider immediately if they experience a seizure.
- Advise patients, especially elderly patients, that cognitive impairment may occur during treatment with scopolamine transdermal system, especially in those receiving other drugs also associated with CNS effects, and to report to their healthcare provider if they develop signs or symptoms of cognitive impairment such as hallucinations, confusion or dizziness.
- Inform patients not to operate motor vehicles or other dangerous machinery or participate in underwater sports until they are reasonably certain that scopolamine transdermal system does not affect them adversely [see Warnings and Precautions (5.2)].
Decreased Gastrointestinal Motility and Urinary Retention
Instruct patients to remove the transdermal system if they develop symptoms of intestinal obstruction (abdominal pain, nausea or vomiting) or any difficulties in urinating [see Warnings and Precautions (5.4)].
Hyperthermia
Inform patients that scopolamine transdermal system can increase body temperature and reduce sweating, which may result in hyperthermia and be exacerbated by exposure to external heat sources or high environmental temperature. Geriatric patients may be more susceptible to these effects. Advise patients if body temperature increases, or they are not sweating in warm environmental conditions, remove the transdermal system, and contact their healthcare provider. Symptoms may persist after removal of the transdermal system [see Warnings and Precautions (5.5)].
Drug Withdrawal/Post-Removal Symptoms
Inform patients that if they remove the scopolamine transdermal system after several days of use, withdrawal symptoms may occur and to seek immediate medical care if they develop severe symptoms after removing scopolamine transdermal system [see Warnings and Precautions (5.6)].
Blurred Vision
Inform patients that temporary dilation of the pupils and blurred vision may occur if scopolamine transdermal system comes in contact with the eyes. Instruct patients to wash their hands thoroughly with soap and water immediately after handling the transdermal system, do not touch the system while wearing it, and wash hands and the application site with soap and water after transdermal system removal [see Dosage and Administration (2.1), see Warnings and Precautions (5.7)].
MRI Skin Burns
Instruct patients to remove the scopolamine transdermal system before undergoing an MRI [see Warnings and Precautions (5.8)].
Manufactured for:
Ingenus Pharmaceuticals, LLC
Orlando, FL 32811
Rev. 05/2025
PM1385
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
Scopolamine
(skoe-POL-a-meen)
Transdermal System
Read this Patient Information before you start using scopolamine transdermal system and each time you get a refill. There may be new information. This information does not take the place of talking to your doctor about your medical condition or your treatment.
What is scopolamine transdermal system?
Scopolamine transdermal system is a prescription medicine used for adults to help prevent:
- nausea and vomiting from motion sickness
- nausea and vomiting from anesthesia or taking opioid pain medicines after surgery
It is not known if scopolamine transdermal system is safe and effective in children.
Who should not use scopolamine transdermal system? Do not use scopolamine transdermal system if you:
- have an eye problem called angle closure glaucoma.
- are allergic to scopolamine, belladonna alkaloids or any of the ingredients in scopolamine transdermal system. See the end of this Patient Information leaflet for a list of the ingredients in scopolamine transdermal system. Ask your doctor if you are not sure.
What should I tell my doctor before using scopolamine transdermal system? Before you use scopolamine transdermal system, tell your doctor about all of your medical conditions, including if you:
- have glaucoma (increased pressure in the eye).
- have a history of seizures or psychosis.
- have problems with your stomach or intestines.
- have trouble urinating.
- are scheduled to have a gastric secretion test.
- have liver or kidney problems.
- are pregnant or plan to become pregnant. It is not known if scopolamine can harm your unborn baby.
- are breastfeeding or plan to breastfeed. Scopolamine can pass into your breast milk. Talk to your doctor about the best way to feed your baby if you use scopolamine transdermal system.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements. Scopolamine transdermal system may affect the way other medicines work, and other medicines may affect how scopolamine transdermal system works. Medicines that you take by mouth may not be absorbed well while you use scopolamine transdermal system.
Especially tell your doctor if you take:
- a sedative, hypnotic, opioid or anxiolytic (medicines that make you sleepy)
- an antidepressant medicine
- an anticholinergic medicine, such as an allergy or cold medicine, a medicine to treat bladder or bowel spasms, certain asthma medicines, or other medicines for motion sickness
Ask your doctor if you are not sure if your medicine is one that is listed above. Know the medicines you take. Keep a list of them and show it to your doctor or pharmacist when you get a new medicine.
How should I use scopolamine transdermal system? - See the detailed Instructions for Use for information about how to use scopolamine transdermal system at the end of this Patient Information leaflet.
- It is important that you apply scopolamine transdermal system exactly as your doctor tells you to.
- Your doctor may change your scopolamine transdermal system dose. Do not change your scopolamine transdermal system dose without talking to your doctor.
- Wear only one scopolamine transdermal system at any time.
- If you use too much scopolamine transdermal system, call your doctor or Poison Help line at 1-800-222-1222, or go to the nearest hospital emergency room right away.
What should I avoid while using scopolamine transdermal system? - You should not drink alcohol while using scopolamine transdermal system. It can increase your chances of having serious side effects.
- You should not drive, operate heavy machinery, or do other dangerous activities until you know how scopolamine transdermal system affects you.
- You should not use scopolamine transdermal system during a Magnetic Resonance Imaging scan (MRI). Remove scopolamine transdermal system before undergoing an MRI. It can cause your skin to burn.
- You should be careful if you use scopolamine transdermal system while you participate in watersports because you may feel lost or confused (disoriented).
- Limit contact with water while swimming and bathing because scopolamine transdermal system may fall off. If scopolamine transdermal system falls off, throw it away and apply a new one on the hairless area behind your other ear.
What are the possible side effects of scopolamine transdermal system?
Scopolamine transdermal system may cause serious side effects, including:
- angle closure glaucoma. If you have open angle glaucoma and use scopolamine transdermal system, remove scopolamine transdermal system and call a doctor right away if you feel pain or discomfort, have blurred vision, or see halos or colored images around lights and reddening of your eyes.
- worsening of seizures. Tell your doctor about any worsening of seizures while using scopolamine transdermal system.
-
an unusual reaction called acute psychosis. Tell your doctor if you have any of these symptoms:
- confusion
- agitation
- rambling speech
- hallucinations (seeing or hearing things that are not there)
- paranoid behaviors and delusions (false belief in something)
- worsening of your preeclampsia during pregnancy. Some pregnant women with severe preeclampsia have had seizures after getting scopolamine by injection in the muscle (intramuscular) or injection in the vein (intravenous).
- difficulty urinating.
- difficulties in food passing from the stomach to the small intestines, which may cause abdominal pain, nausea or vomiting.
- increased body temperature (hyperthermia) and a decrease in sweating. If you have a high body temperature or if you are not sweating in warm conditions, remove the transdermal system and contact your doctor. Elderly people may be at greater risk for these side effects.
- withdrawal symptoms after removing scopolamine transdermal system after using it for several days. Some people may have certain symptoms such as difficulty with balance, dizziness, nausea, vomiting, stomach cramps, sweating, confusion, muscle weakness, low heart rate or low blood pressure that could start 24 hours or more after removing scopolamine transdermal system. Call your doctor right away if your symptoms become severe.
- temporary increase in the size of your pupil and blurry vision, especially if scopolamine transdermal system comes in contact with your eyes.
- skin burns at the site of scopolamine transdermal system. This can happen during a medical test called a Magnetic Resonance Imaging scan (MRI). Scopolamine transdermal system contains aluminum and should be removed from your skin before you have an MRI.
The most common side effects of using scopolamine transdermal system include:
dry mouth blurred vision or eye problems feeling sleepy or drowsy disorientation (confusion)
dizziness feeling agitated or irritable pharyngitis (sore throat)
Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of scopolamine transdermal system.Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of scopolamine transdermal system.
Medicines are sometimes prescribed for purposes other than those listed in a patient information leaflet. Do not use scopolamine transdermal system for a condition for which it was not prescribed. Do not give scopolamine transdermal system to other people, even if they have the same symptoms you have. It may harm them.
You can ask your pharmacist or doctor for information about scopolamine transdermal system that is written for health professionals.
What are the ingredients in scopolamine transdermal system?
Active ingredient: scopolamine
Inactive ingredients: Aluminized polyester backing film, acrylic adhesive, siliconized PET release liner, ethylene vinyl acetate membrane and isopropyl myristate. The brown imprinting ink contains FD&C yellow no. 6 aluminum lake, polyamide resin, polytetrafluoroethylene, polyethylene wax, carbon black and quinacridone red pigment.
For more information, call Ingenus Pharmaceuticals, LLC at 1-877-748-1970.
-
INSTRUCTIONS FOR USE
Scopolamine (skoe-POL-a-meen) Transdermal System
Read this Instructions for Use before you start using scopolamine transdermal system and each time you get a refill. There may be new information. This information does not take the place of talking to your doctor about your medical condition or your treatment.
Information about scopolamine transdermal system:
- Scopolamine transdermal system is a tan-colored, circle shaped transdermal system (patch) with “Scopolamine 1 mg/3 days” printed on it.
- Wear only one scopolamine transdermal system at any time.
- Do not cut the scopolamine transdermal system.
To help prevent nausea and vomiting from motion sickness:
- Apply one scopolamine transdermal system to your skin on a hairless area behind one ear at least 4 hours before the activity to prevent nausea and vomiting.
- If the treatment is needed for longer than 3 days, remove scopolamine transdermal system from the hairless area behind your ear. Get a new scopolamine transdermal system and place it on the hairless area behind your other ear.
To help prevent nausea and vomiting after surgery:
- Follow your doctor’s instructions about when to apply scopolamine transdermal system before your scheduled surgery.
- Scopolamine transdermal system should be left in place for 24 hours after surgery. After 24 hours, scopolamine transdermal system should be removed and thrown away.
How to use scopolamine transdermal system:
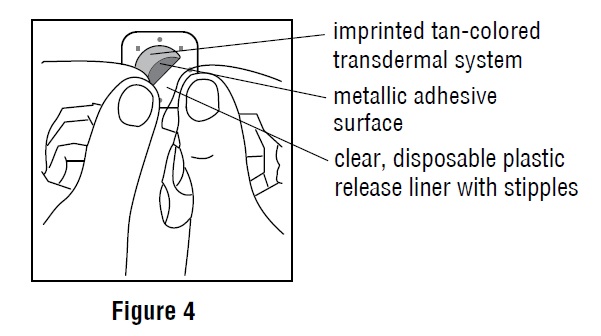
Inside the scopolamine transdermal system package, you will find one scopolamine transdermal system. An imprinted, tan backing membrane with a metallic (silver) sticky surface is adhered to a clear, disposable plastic release liner with stipples (See Figure 1).
1. Select a hairless area of skin behind one of your ears. Avoid areas on your skin that may have cuts, pain or tenderness. Wipe the area of your skin with a clean, dry tissue.

2. Tear along the dashed line on the scopolamine transdermal system package to open (See Figure 2).
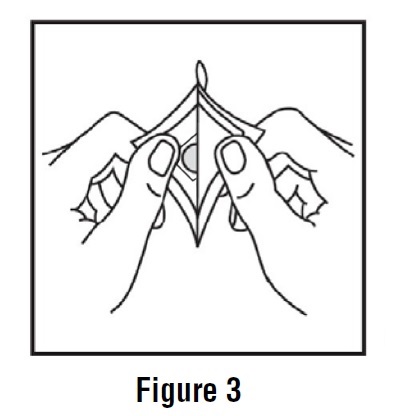
3. Remove the clear plastic backing (disposable release liner with stipples) from the tan-colored round scopolamine transdermal system (See Figure 3).
4. Do not touch the metallic adhesive (sticky) surface on scopolamine transdermal system with your hands (See Figure 4).
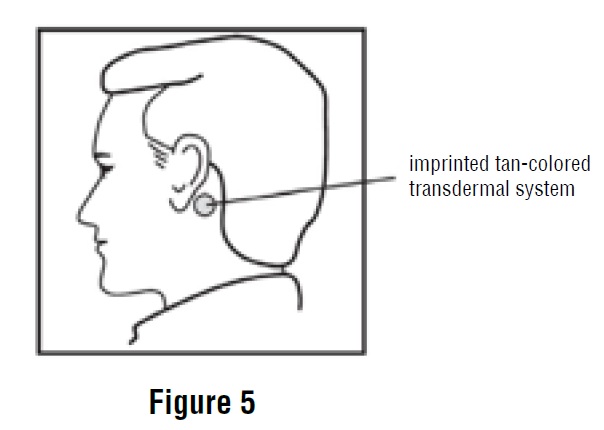
5. Apply the metallic adhesive surface of scopolamine transdermal system firmly to the dry area of skin behind your ear. The imprinted, tan-colored side of the scopolamine transdermal system should be facing up and showing (See Figure 5). After placement of scopolamine transdermal system, avoid touching or applying pressure to the transdermal system while it is being worn because applying pressure may cause scopolamine to ooze out at the edge. Wash your hands with soap and water right away after applying scopolamine transdermal system, so that any medicine from the scopolamine transdermal system that gets on your hands will not get into your eyes.
How to remove scopolamine transdermal system:
After removing scopolamine transdermal system, be sure to wash your hands and the area behind your ear thoroughly with soap and water. Please note that the used scopolamine transdermal system will still contain some of the active ingredient after use. To avoid accidental contact or ingestion by children, pets or others, fold the used scopolamine transdermal system in half with the sticky side together. Throw away (dispose of) scopolamine transdermal system in the household trash out of the reach of children, pets or others.
How should I store scopolamine transdermal system?
- Store scopolamine transdermal system at room temperature between 68°F to 77°F (20°C to 25°C) until you are ready to use it.
- Store scopolamine transdermal system in an upright position.
- Do not bend or roll scopolamine transdermal system
Keep scopolamine transdermal system and all medicines out of reach of children.
The Patient Information and Instructions for Use have been approved by the U.S. Food and Drug Administration.
Manufactured for:
Ingenus Pharmaceuticals, LLC
Orlando, FL 32811
Rev. 05/2025
Rev-04
-
PRINCIPAL DISPLAY PANEL
Package Label - Principal Display Panel – 1 Patch
ingenus
NDC: 50742-505-01
Scopolamine Transdermal System
1 mg/3 days
CAUTION: WASH HANDS IMMEDIATELY AFTER APPLICATION.
CONTACT WITH EYES MAY CAUSE IRRITATION.May cause drowsiness, blurred vision. To avoid possible burns, remove scopolamine transdermal system before undergoing an MRI (Magnetic Resonance Imaging) procedure.
Rx only
Contents: 1 Transdermal System
Front

Back
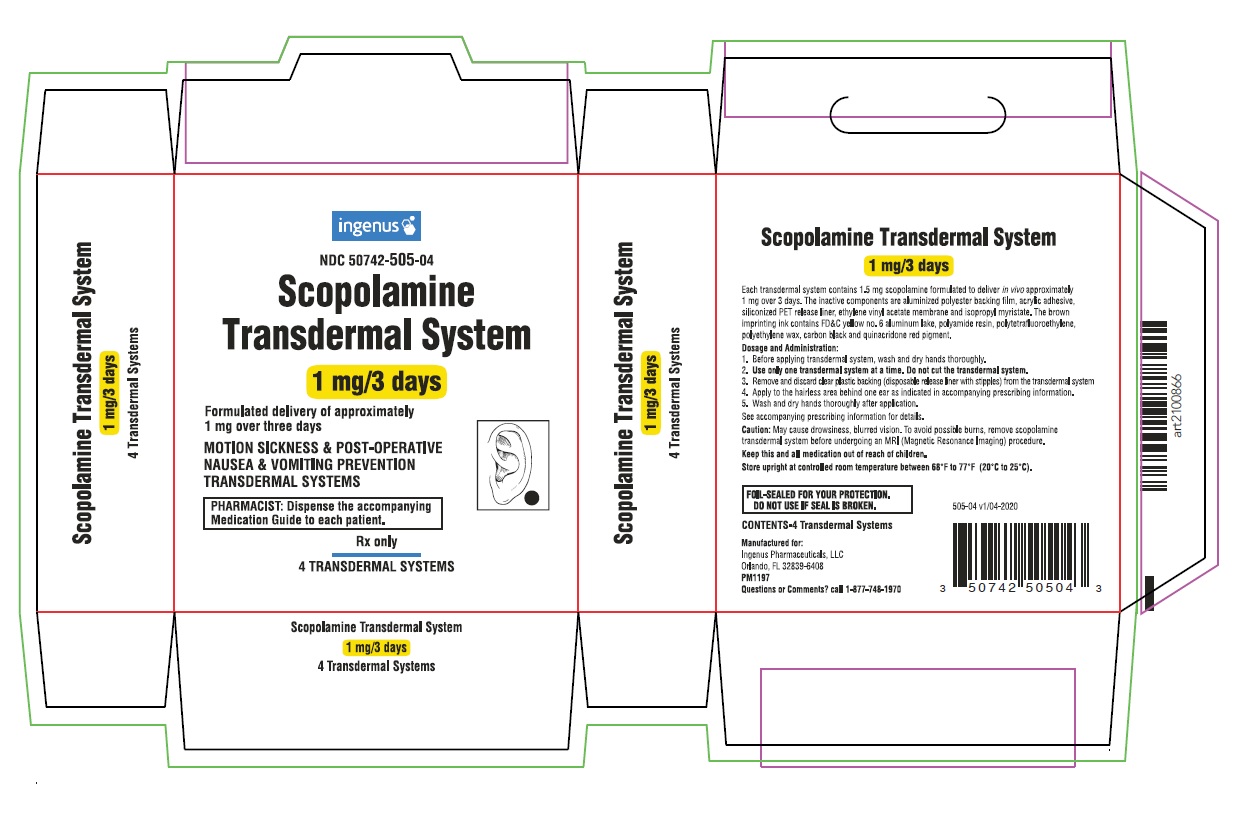
-
PRINCIPAL DISPLAY PANEL
ingenus
NDC: 50742-505-04
Scopolamine Transdermal System
1 mg/3 days
Formulated delivery of approximately 1 mg over three days
Rx only
4 TRANSDERMAL SYSTEMS MULTIPACK

-
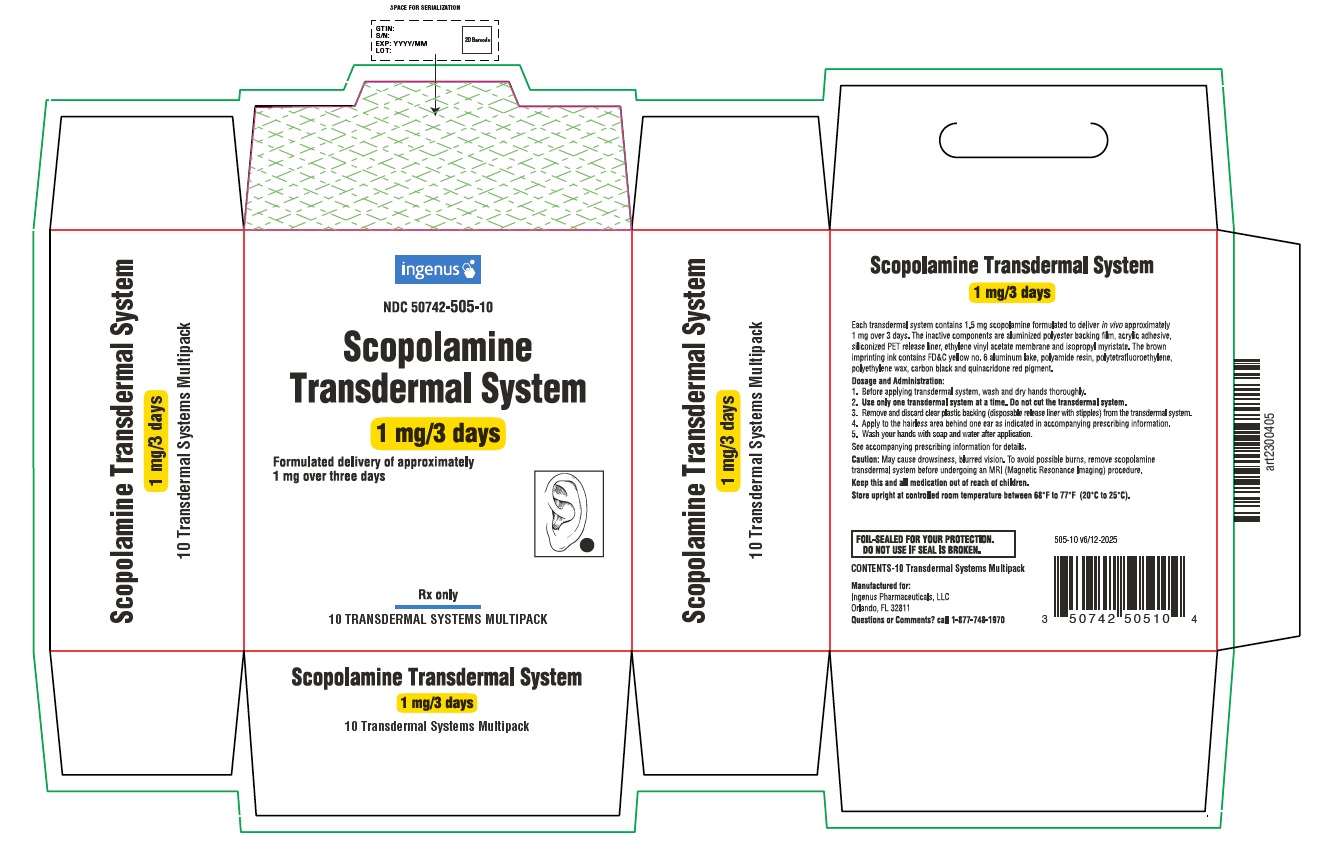
PRINCIPAL DISPLAY PANEL
ingenus
NDC: 50742-505-10
Scopolamine Transdermal System
1 mg/3 days
Formulated delivery of approximately 1 mg over three days
Rx only
10 TRANSDERMAL SYSTEMS MULTIPACK
-
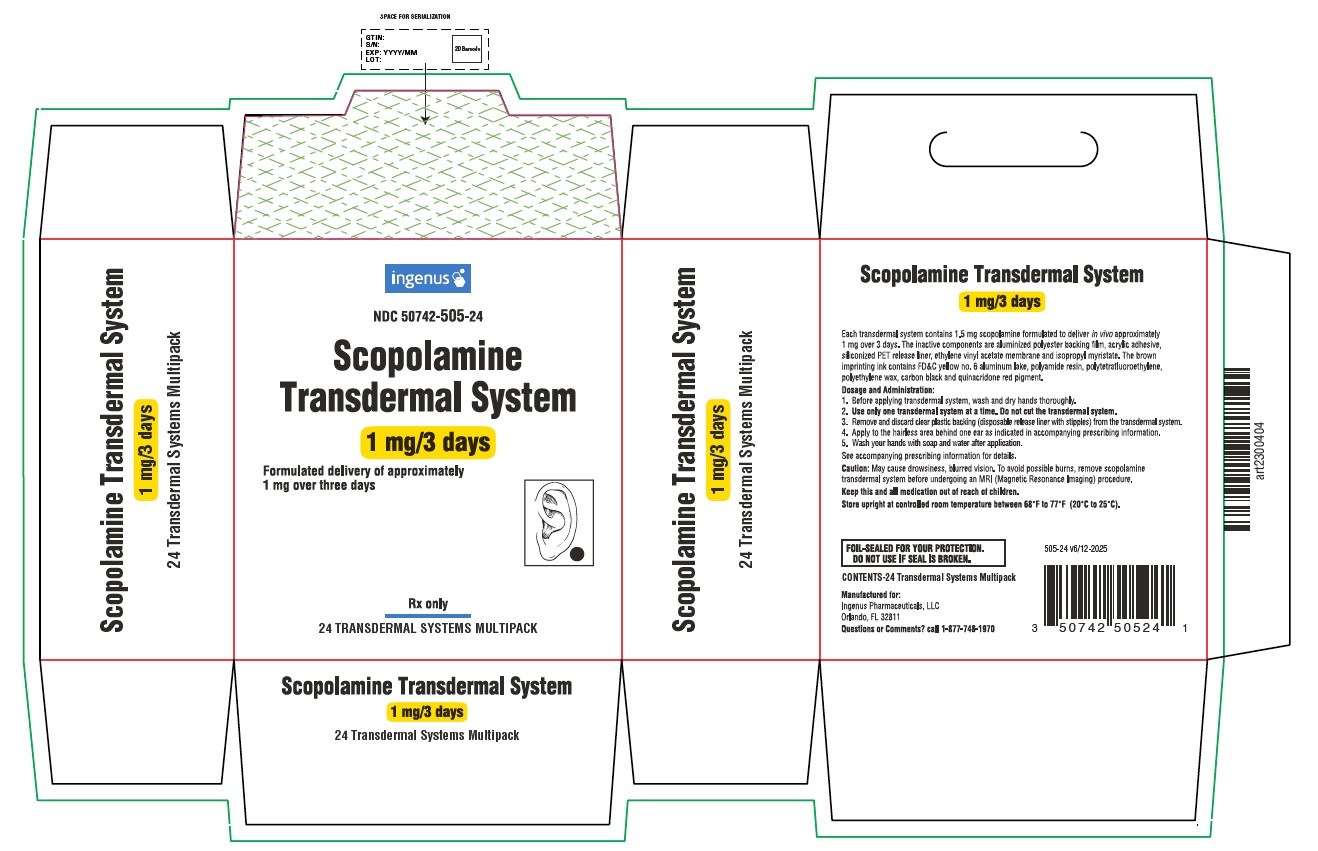
PRINCIPAL DISPLAY PANEL
ingenus
NDC: 50742-505-24
Scopolamine Transdermal System
1 mg/3 days
Formulated delivery of approximately 1 mg over three days
Rx only
24 TRANSDERMAL SYSTEMS MULTIPACK
-
INGREDIENTS AND APPEARANCE
SCOPOLAMINE
scopolamine patch, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 50742-505 Route of Administration TRANSDERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SCOPOLAMINE (UNII: DL48G20X8X) (scopolamine - UNII:DL48G20X8X) SCOPOLAMINE 1.5 mg Inactive Ingredients Ingredient Name Strength ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ETHYL ACETATE (UNII: 76845O8NMZ) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) POLYTETRAFLUOROETHYLENE (UNII: E1NC1JVS3O) D&C BLACK NO. 2 (UNII: 4XYU5U00C4) CINQUASIA RED (UNII: 11P487375P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50742-505-04 4 in 1 CARTON 11/24/2020 1 1 in 1 POUCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC: 50742-505-10 10 in 1 CARTON 11/24/2020 2 1 in 1 POUCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 3 NDC: 50742-505-24 24 in 1 CARTON 11/24/2020 3 1 in 1 POUCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA212342 11/24/2020 Labeler - Ingenus Pharmaceuticals, LLC (833250017) Registrant - RICONPHARMA LLC (859035318) Establishment Name Address ID/FEI Business Operations Surmasis Pharmaceutical 079203494 manufacture(50742-505)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.