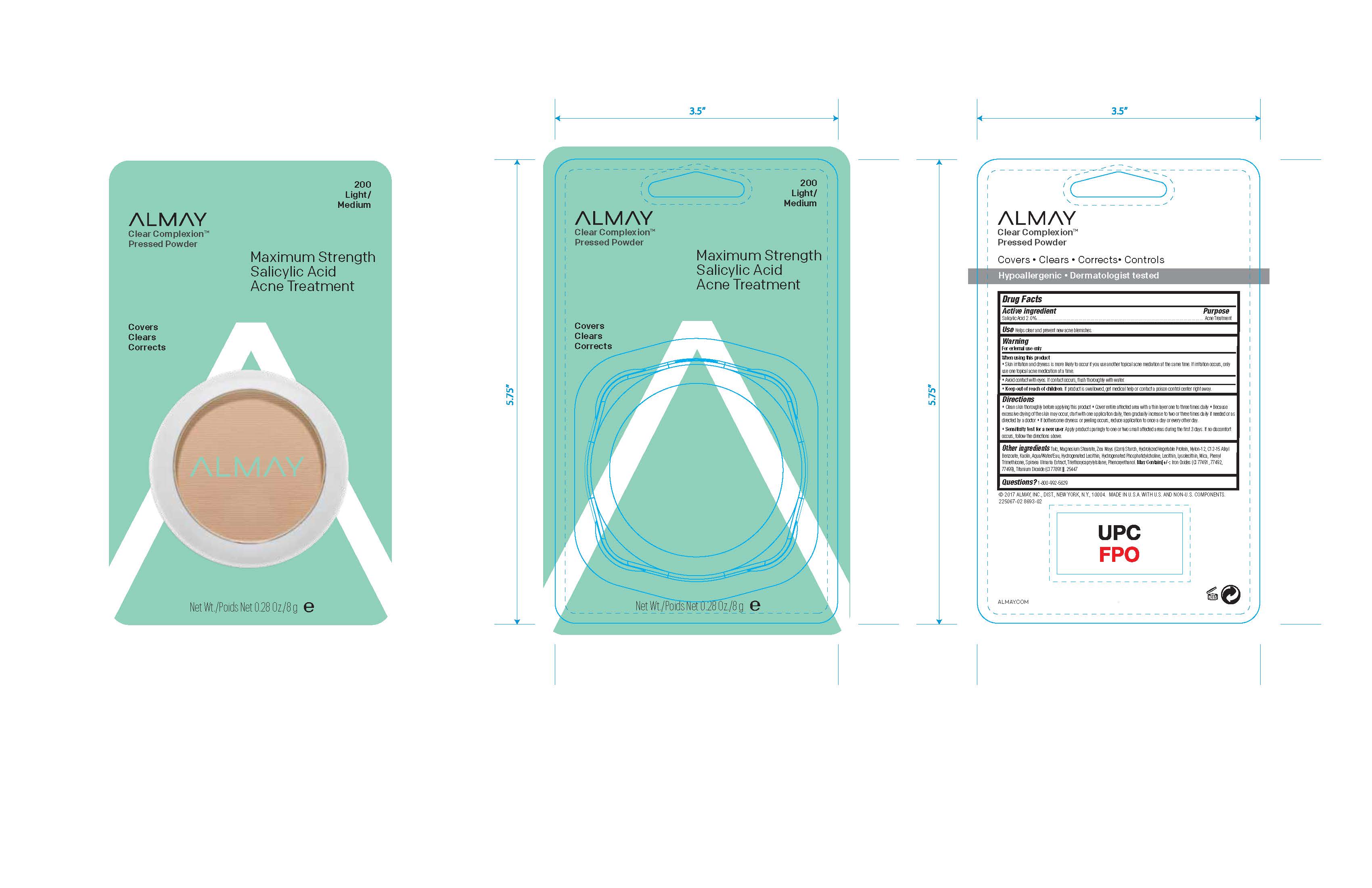
Almay Clear Complexion Pressed Powder
Almay Clear Complexion Pressed Powder by
Drug Labeling and Warnings
Almay Clear Complexion Pressed Powder by is a Otc medication manufactured, distributed, or labeled by Almay, Inc., REVLON, INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ALMAY CLEAR COMPLEXION PRESSED POWDER MEDIUM-DARK- salicylic acid powder
Almay, Inc.
----------
Almay Clear Complexion Pressed Powder
Warnings:
For external use only.
Skin irritation and dryness is likely to occur if you use antother topica; acne medication at the same time. If irritation occurs, use only one topical acne medication at a time.
Avoid contact with eyes. If contact occurs, flush throughly with water.
Directions
Clean skin thoroughly
before applying this product Cover the
entire affected area with a thin layer 1 to 3
times daily. Because excessive drying of
the skin may occur, start with 1 appli -
cation daily, then gradually increase to 2
or 3 times daily if needed or as directed
by doctor. If bothersome dryness or
peeling occurs, reduce application to once
a day or every other day.
Inactive Ingredients
Talc, Magnesium Stearate, Zea Mays (Corn) Starch, Hydrolyzed Vegetable Protein, Nylon-12, C12-15 Alkyl
Benzoate, Kaolin, Aqua/Water/Eau, Hydrogenated Lecithin, Hydrogenated Phosphatidylcholine, Lecithin, Lysolecithin, Mica, Phenyl
Trimethicone, Spiraea Ulmaria Extract, Triethoxycaprylylsilane, Phenoxyethanol. May Contain[+/-: Iron Oxides (CI 77491, 77492,
77499), Titanium Dioxide (CI 77891)].
| ALMAY CLEAR COMPLEXION PRESSED POWDER
MEDIUM-DARK
salicylic acid powder |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Almay, Inc. (064988652) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| REVLON, INC. | 809725570 | manufacture(0311-0729) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.