SPOT REMOVER ACNE TREATMENT PADS- salicylic acid liquid
SPOT REMOVER ACNE TREATMENT PADS by
Drug Labeling and Warnings
SPOT REMOVER ACNE TREATMENT PADS by is a Otc medication manufactured, distributed, or labeled by ORIGINS NATURAL RESOURCES INC., ESTEE LAUDER COMPANY, THE, ESTEE LAUDER COSMETICS, LTD, ESTEE LAUDER COSMETICS, LTD., ESTEE LAUDER N.V., LEN-RON MANUFACTURING DIVISION OF ARAMIS INC, NORTHTEC INC, PADC 1, WHITMAN LABORATORIES, LTD.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
-
Inactive ingredients
water\aqua\eau alcohol denat. peg-60 almond glycerides betaine eugenia caryophyllus (clove) bud oil1, origanum heracleoticum flower oil1, eugenol boswellia serrata extract serenoa serrulata (saw palmetto) fruit extract phyllanthus emblica fruit extract caffeine sodium hydroxide <iln37564>
- 1 essential oil
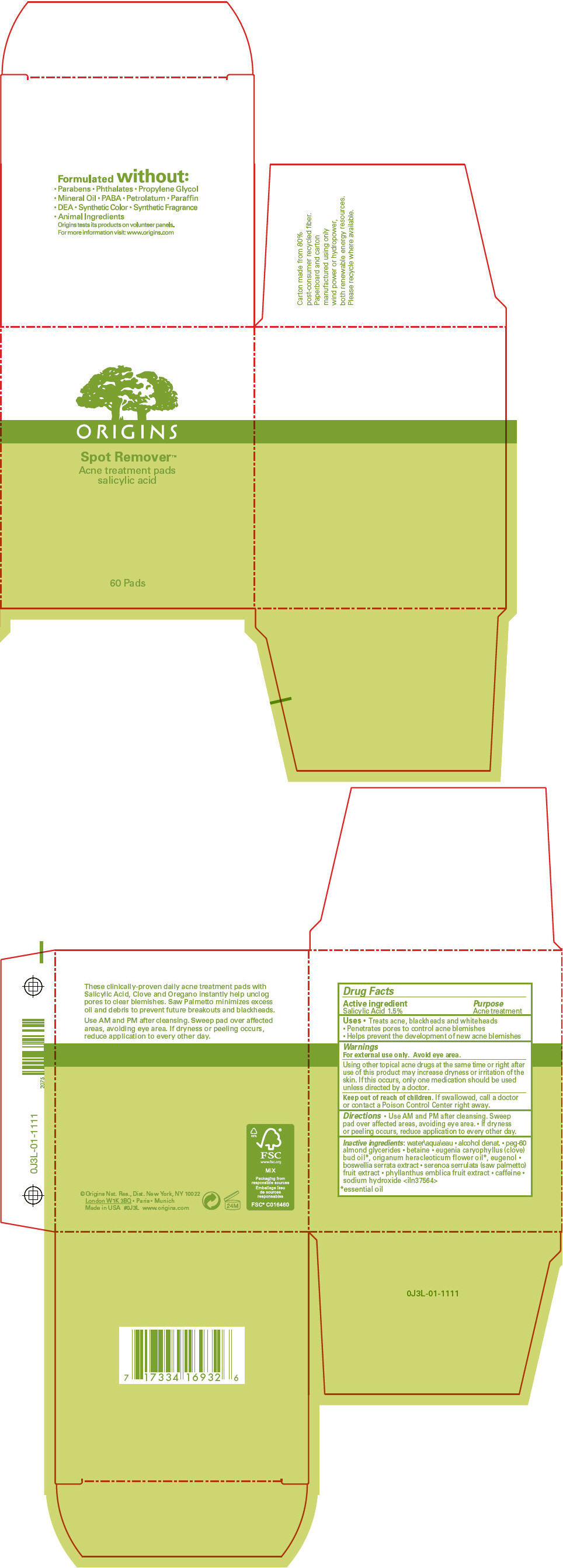
- PRINCIPAL DISPLAY PANEL - 60 Pad Jar Carton
-
INGREDIENTS AND APPEARANCE
SPOT REMOVER ACNE TREATMENT PADS
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 59427-110 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 0.009506 g in 100 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) peg-60 almond glycerides (UNII: 4Y0E651N0F) betaine (UNII: 3SCV180C9W) clove oil (UNII: 578389D6D0) eugenol (UNII: 3T8H1794QW) indian frankincense (UNII: 4PW41QCO2M) saw palmetto (UNII: J7WWH9M8QS) phyllanthus emblica fruit (UNII: YLX4CW2576) caffeine (UNII: 3G6A5W338E) sodium hydroxide (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59427-110-01 60 in 1 JAR 04/01/2011 1 1 mL in 1 APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part333D 04/01/2011 Labeler - ORIGINS NATURAL RESOURCES INC. (611716283) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COMPANY, THE 828534516 RELABEL(59427-110) , REPACK(59427-110) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COSMETICS, LTD 244669714 MANUFACTURE(59427-110) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COSMETICS, LTD. 202952982 MANUFACTURE(59427-110) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER N.V. 370151326 MANUFACTURE(59427-110) Establishment Name Address ID/FEI Business Operations LEN-RON MANUFACTURING DIVISION OF ARAMIS INC 809771152 MANUFACTURE(59427-110) Establishment Name Address ID/FEI Business Operations NORTHTEC INC 943871157 RELABEL(59427-110) , REPACK(59427-110) Establishment Name Address ID/FEI Business Operations PADC 1 949264774 RELABEL(59427-110) , REPACK(59427-110) Establishment Name Address ID/FEI Business Operations WHITMAN LABORATORIES, LTD. 216866277 MANUFACTURE(59427-110) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COSMETICS, LTD 255175580 MANUFACTURE(59427-110)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.