Pipette Eczema by Pipette LLC / Northwest Cosmetic Laboratories L.L.C. DBA Elevation Labs, Idaho Pipette Eczema Lotion
Pipette Eczema by
Drug Labeling and Warnings
Pipette Eczema by is a Otc medication manufactured, distributed, or labeled by Pipette LLC, Northwest Cosmetic Laboratories L.L.C. DBA Elevation Labs, Idaho. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
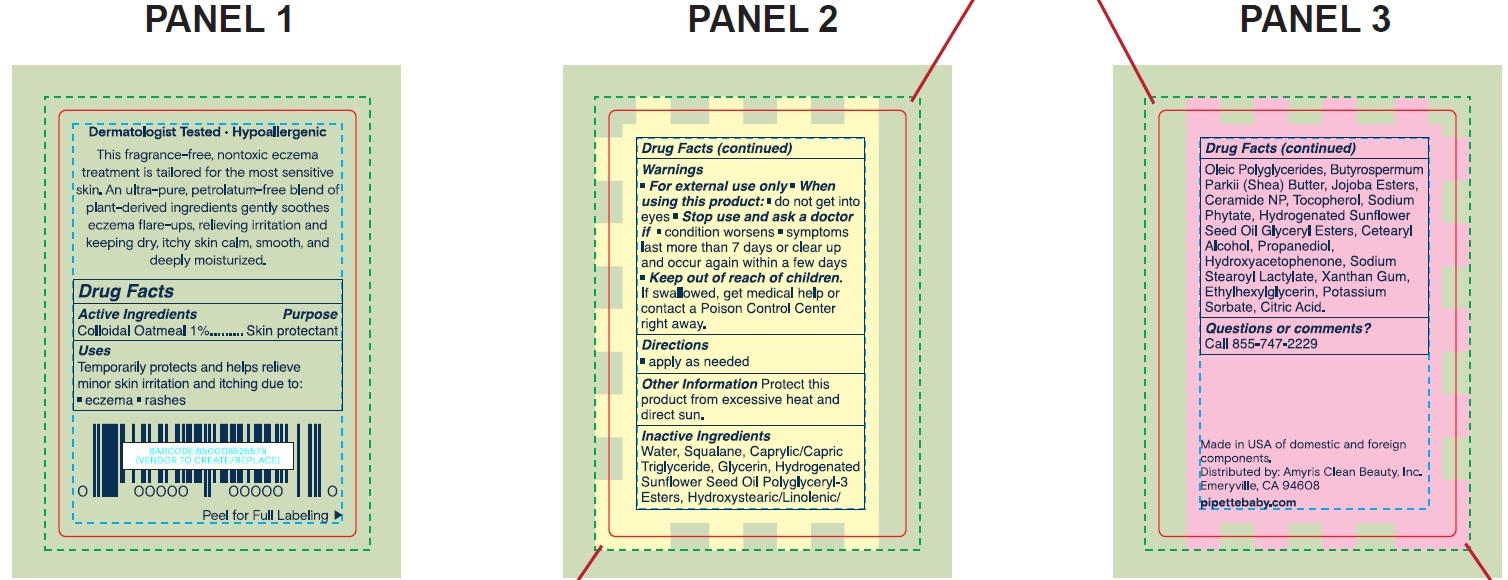
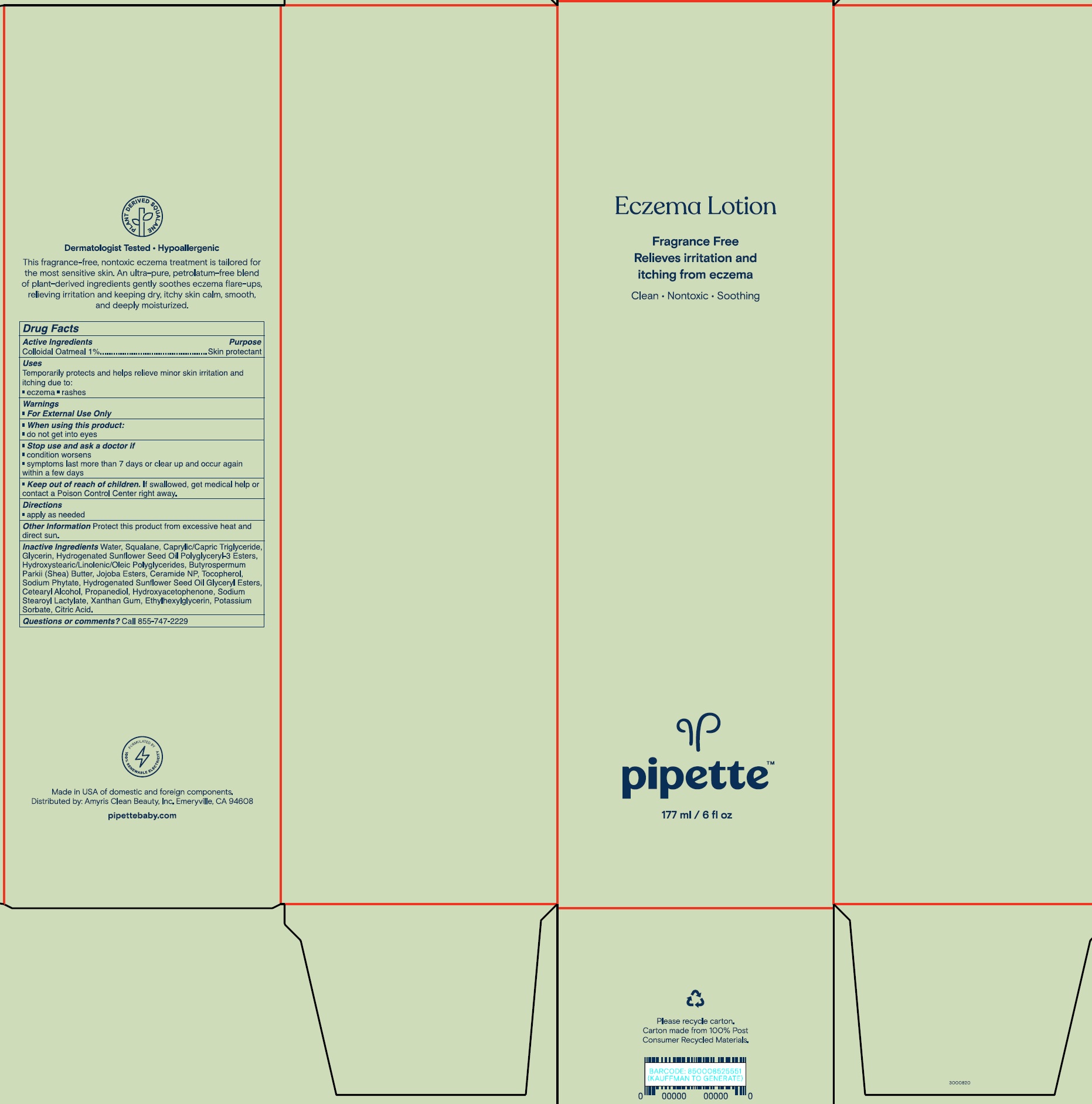
PIPETTE ECZEMA- colloidal oatmeal lotion
Pipette LLC
----------
Pipette Eczema Lotion
Warnings
- For external use only
Inactive Ingredients
Water, Squalane, Caprylic/Capric Triglyceride, Glycerin, Hydrogenated Sunflower Seed Oil Polyglyceryl-3 Esters, Hydroxystearic/Linolenic/Oleic Polyglycerides, Butyrospermum Parkii (Shea) Butter, Jojoba Esters, Ceramide NP, Tocopherol, Sodium Phytate, Hydrogenated Sunflower Seed Oil, Glyceryl Esters, Cetearyl Alcohol, Propanediol, Hydroxyacetophenone, Sodium Stearoyl Lactylate, Xanthan Gum, Ethylhexylglycerin, Potassium Sorbate, Citric Acid.
| PIPETTE ECZEMA
colloidal oatmeal lotion |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - Pipette LLC (119210873) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Northwest Cosmetic Laboratories L.L.C. DBA Elevation Labs, Idaho | 929572014 | manufacture(84723-003) | |
Revised: 7/2025
Document Id: 398325f0-ff46-f480-e063-6294a90abff0
Set id: 2a939145-4525-c9af-e063-6294a90a0f34
Version: 2
Effective Time: 20250709
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.