Sterile Diluent for M-M-R ®II (Measles, Mumps, and Rubella Virus Vaccine Live) Suspension for intramuscular or subcutaneous injection Initial U.S. Approval: 1978 These highlights do not include all the information needed to use M-M-R II safely and effectively. See full prescribing information for M-M-R II.
Sterile Diluent for M-M-R II by
Drug Labeling and Warnings
Sterile Diluent for M-M-R II by is a Other medication manufactured, distributed, or labeled by Bamboo US BidCo LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
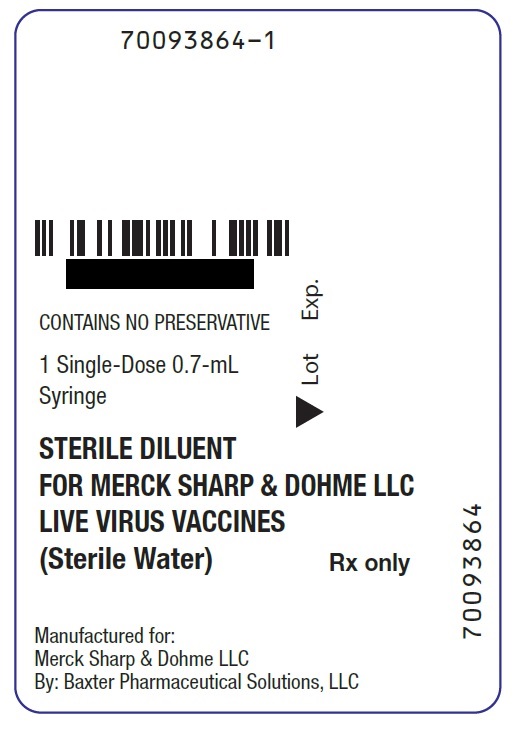
STERILE DILUENT FOR M-M-R II- measles, mumps, and rubella virus vaccine live injection, powder, lyophilized, for suspension
Bamboo US BidCo LLC
----------
Sterile Diluent for M-M-R
®II (Measles, Mumps, and Rubella Virus Vaccine Live) Suspension for intramuscular or subcutaneous injection
Initial U.S. Approval: 1978
These highlights do not include all the information needed to use M-M-R II safely and effectively. See full prescribing information for M-M-R II.
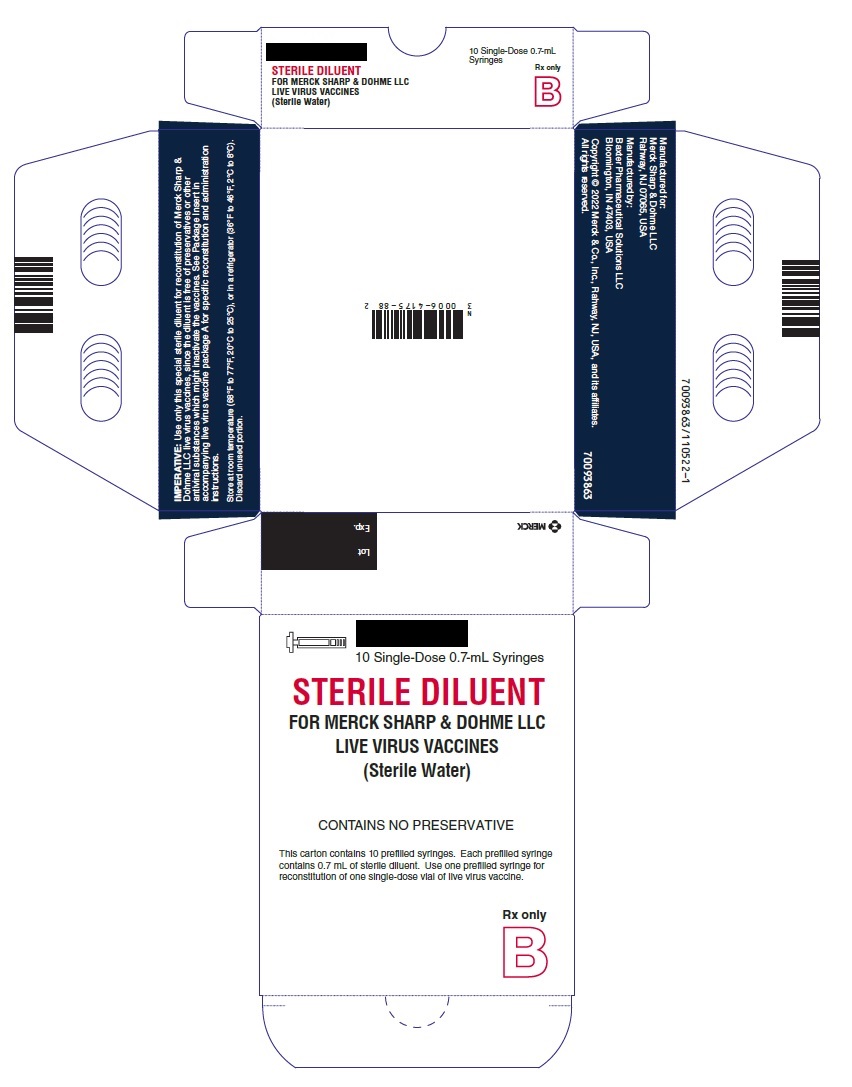
PRINCIPAL DISPLAY PANEL - 0.7 mL Syringe
10 Single-Dose 0.7-mL Syringes
STERILE DILUENT
FOR MERCK SHARP & DOHME LLC
LIVE VIRUS VACCINES
(Sterile Water)
CONTAINS NO PRESERVATIVE
This carton contains 10 prefilled syringes. Each prefilled syringe
contains 0.7 mL of sterile diluent. Use one prefilled syringe for
reconstitution of one single-dose vial of live virus vaccine.
Rx only
| STERILE DILUENT FOR M-M-R II
measles, mumps, and rubella virus vaccine live injection, powder, lyophilized, for suspension |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Bamboo US BidCo LLC (119087615) |
Revised: 6/2025
Document Id: 387edfd5-8290-0f50-e063-6394a90aef47
Set id: 2abf71cb-5c02-c5af-e063-6394a90a34b1
Version: 2
Effective Time: 20250626
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.