XAFVRQ Hair Regrowth Treatment by COMART TECH LLC
XAFVRQ Hair Regrowth Treatment by
Drug Labeling and Warnings
XAFVRQ Hair Regrowth Treatment by is a Otc medication manufactured, distributed, or labeled by COMART TECH LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
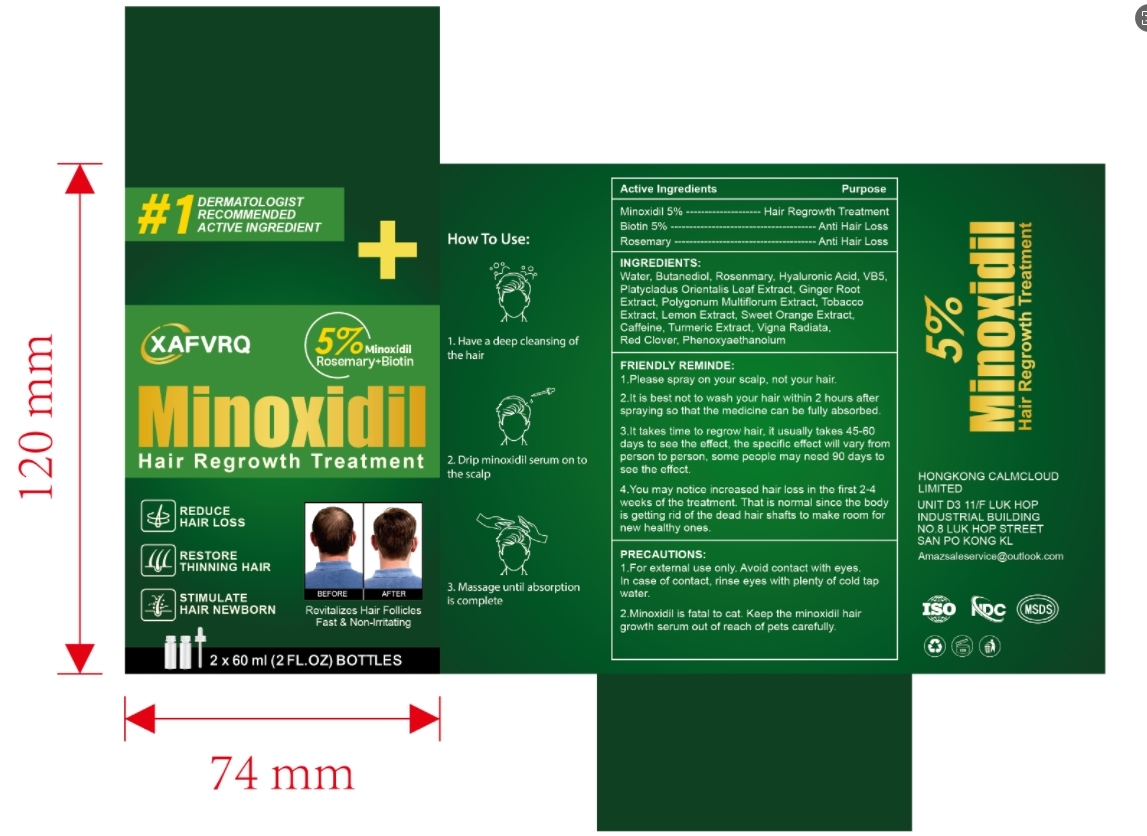
XAFVRQ HAIR REGROWTH TREATMENT- minoxidil wiht biotin 5% liquid
COMART TECH LLC
----------
1. Massage the scalp to fully relax the scalp.
2. Ad one or two drops to the scalp area to be used.
3. Apply evenly to the rest of the area.
4. Continue to use weice a day.
1. Do not apply on other parts of the body, For topical use only on the scalp.
2. Avoid contact with the eyes in case of accidental contact rinse eyes with large arnounts of cool tap water.
3. Some people have experienced changes in hair color and/or texture.
4. The amount of hair regrowth is dlfferent for each person. This product will not work for all men.
Usage:1. Massage the scalp to fully relax the scalp.2. Ad one or two dropsto the scalp area to be used.3. Apply evenly to the rest of the area.4. Continue to use weice a day.
Friendly reminder: it takes time to regrow hair, it usually takes 45-60 days to see the effect, the speciflc effect will vary from person to person, some people may need 90 days to see the effect.
You have no family history of hair loss,hair loss is sudden and/or patchy.
You do not know the reason for you hair loss.
You ae under 18 years of age, Do not use it on babies and children your scalp is red,inflamed,infected, irritated, or painful.
You use other medicines on the scalp.Ask a doctor before use if you hace heart disease.
Chest pain, rapid heart beat, faintness, or dizziness occurs sudden, unexplained weight gain occurs,your hands or feet swell.
INGREDIENTS:
Water, Butanediol, Rosenmary, Hyaluronic Acid, VB5,Platycladus Orientalis Leaf Extract, Ginger Root Extract, Polygonum Multiflorum Extract, TobaccoExtract, Lemon Extract, Sweet Orange Extract.Caffeine, Turmeric Extract, Vigna Radiata,Red Clover, Phenoxyaethanolum
INGREDIENTS:
Water, Butylene Glycol, Rosmarinus Officinalis (rosemary) Extract, Hyaluronic Acid, VB5,Platycladus Orientalis Leaf Extract, Ginger RootExtract, Polygonum Multiflorum Extract, TobaccoExtract, Lemon Extract, Sweet Orange Extract,Caffeine, Turmeric Extract, Vigna Radiata,Red Clover, Phenoxyaethanolum
FRIENDLY REMINDE
1.Please spray on your scalp, not your hair.2.lt is best not to wash your hair within 2 hours afterspraying so that the medicine can be fully absorbed
3.lt takes time to regrow hair, it usually takes 45-60days to see the effect, the specific effect will vary fromperson to person, some people may need 90 days tosee the effect.
4.You may notice increased hair loss in the first 2-4weeks of the treatment. That is normal since the bodyis getting rid of the dead hair shafts to make room fornew healthy ones
| XAFVRQ HAIR REGROWTH TREATMENT
minoxidil wiht biotin 5% liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - COMART TECH LLC (131800982) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.