ERVEBO (zaire ebolavirus- strain kikwit-95 envelope glycoprotein injection, solution
ERVEBO by
Drug Labeling and Warnings
ERVEBO by is a Other medication manufactured, distributed, or labeled by Merck Sharp & Dohme LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ERVEBO safely and effectively. See full prescribing information for ERVEBO.
ERVEBO® (Ebola Zaire Vaccine, Live) Suspension for intramuscular injection
Initial U.S. Approval: 2019RECENT MAJOR CHANGES
Indications and Usage (1) 07/2023 INDICATIONS AND USAGE
ERVEBO® is a vaccine indicated for the prevention of disease caused by Zaire ebolavirus in individuals 12 months of age and older. (1)
Limitations of Use (1.1)
- The duration of protection conferred by ERVEBO is unknown.
- ERVEBO does not protect against other species of Ebolavirus or Marburgvirus.
- Effectiveness of the vaccine when administered concurrently with antiviral medication, immune globulin (IG), and/or blood or plasma transfusions is unknown.
DOSAGE AND ADMINISTRATION
- Administer a single 1 mL dose of ERVEBO intramuscularly. (2.1)
DOSAGE FORMS AND STRENGTHS
- 1 mL suspension for injection supplied as a single-dose vial. (3)
CONTRAINDICATIONS
- Severe allergic reaction (e.g., anaphylaxis) to any component of ERVEBO. (4)
WARNINGS AND PRECAUTIONS
- Anaphylaxis has been observed following administration of ERVEBO. Appropriate medical treatment and supervision must be available in case of anaphylactic event following the administration of ERVEBO. (5.1)
- Vaccinated individuals should continue to adhere to infection control practices to prevent Zaire ebolavirus infection and transmission. (5.2)
- Vaccine virus RNA has been detected in blood, saliva, urine, and fluid from skin vesicles of vaccinated individuals; transmission of vaccine virus is a theoretical possibility. (5.4)
ADVERSE REACTIONS
The most commonly reported local and systemic adverse events in clinical trials were:
- Individuals 18 years of age and older: injection-site pain (70%); headache (55%); feverishness (39%); muscle pain (33%); somnolence, reduced activity, fatigue (26%); joint pain, arthralgia (19%); chills (17%); injection-site swelling (17%); decreased appetite (15%); abdominal pain (13%); injection-site redness (12%); nausea (10%); arthritis (5%); vomiting (4%), rash (4%); abnormal sweating (3%) and mouth ulceration (2%). (6.1)
- Individuals 12 months through 2 years of age: feverishness (83%); crying (31%); decreased appetite (27%); injection-site pain (26%); somnolence, reduced activity, fatigue (20%); diarrhea (19%); vomiting (17%); irritability (11%); screaming (10%); mouth ulceration (6%); chills (5%); injection-site swelling (5%); headache (4%); abdominal pain (2%); abnormal sweating (2%) and injection-site erythema (1%). (6.1)
- Individuals 3 years through 11 years of age: feverishness (65%); headache (50%); injection-site pain (40%); decreased appetite (24%); somnolence, reduced activity, fatigue (22%); abdominal pain (21%); chills (14%); myalgia (12%); vomiting (11%);dizziness (8%); nausea (8%); injection-site pruritus (7%); crying (3%); arthralgia (3%); diarrhea (3%); injection-site swelling (3%); abnormal sweating (1%); mouth ulceration (2%) and irritability (1%). (6.1)
- Individuals 12 years through 17 years of age: headache (59%); injection-site pain (52%); feverishness (48%); myalgia (30%); somnolence, reduced activity, fatigue (28%); decreased appetite (21%); chills (19%); dizziness (17%); abdominal pain (16%); arthralgia (16%); nausea (8%); abnormal sweating (5%); diarrhea (4%); vomiting (4%); injection-site pruritus (3%); injection-site swelling (3%) and mouth ulceration (2%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Merck Sharp & Dohme LLC at 1-877-888-4231 or VAERS at 1-800-822-7967 or www.vaers.hhs.gov.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 7/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Limitations of Use
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
2.2 Preparation
2.3 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Management of Acute Allergic Reactions
5.2 Limitations of Vaccine Effectiveness
5.3 Immunocompromised Individuals
5.4 Transmission
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Interference with Laboratory Tests
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Clinical Efficacy
14.2 Clinical Immunogenicity
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
ERVEBO® is indicated for the prevention of disease caused by Zaire ebolavirus in individuals 12 months of age and older.
1.1 Limitations of Use
- The duration of protection conferred by ERVEBO is unknown.
- ERVEBO does not protect against other species of Ebolavirus or Marburgvirus.
- Effectiveness of the vaccine when administered concurrently with antiviral medication, immune globulin (IG), and/or blood or plasma transfusions is unknown.
-
2 DOSAGE AND ADMINISTRATION
FOR INTRAMUSCULAR ADMINISTRATION ONLY.
2.2 Preparation
Thaw vial at room temperature until no visible ice is present. Do not thaw the vial in a refrigerator. Gently invert vial several times. The vaccine is a colorless to slightly brownish-yellow liquid with no particulates visible. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If either of these conditions exist, discard the vial.
Use the vaccine immediately after thawing. If not used immediately, the vaccine may be stored for 4 hours at room temperature (up to 25°C; 77°F) protected from light. DO NOT REFREEZE [see How Supplied/Storage and Handling (16)].
Withdraw the 1 mL dose of vaccine from the vial using a sterile needle and sterile syringe.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Do not administer ERVEBO to individuals with a history of a severe allergic reaction (e.g., anaphylaxis) to any component of the vaccine, including rice protein [see Description (11)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Management of Acute Allergic Reactions
Among 18,616 participants vaccinated with at least one dose of ERVEBO in clinical trials, there were two reports of anaphylaxis [see Adverse Reactions (6.1)]. Monitor individuals for signs and symptoms of hypersensitivity reactions following vaccination with ERVEBO. Appropriate medical treatment and supervision must be available in case of an anaphylactic event following the administration of ERVEBO.
5.2 Limitations of Vaccine Effectiveness
Vaccination with ERVEBO may not protect all individuals. Vaccinated individuals should continue to adhere to infection control practices to prevent Zaire ebolavirus infection and transmission.
5.3 Immunocompromised Individuals
The safety and effectiveness of ERVEBO have not been assessed in immunocompromised individuals. The effectiveness of ERVEBO in immunocompromised individuals may be diminished. The risk of vaccination with ERVEBO, a live virus vaccine, in immunocompromised individuals should be weighed against the risk of disease due to Zaire ebolavirus.
5.4 Transmission
Vaccine virus RNA has been detected by RT-PCR in blood, saliva, urine, and fluid from skin vesicles of vaccinated individuals. Transmission of vaccine virus is a theoretical possibility [see Pharmacokinetics (12.3)].
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared to rates in the clinical trials of another vaccine and may not reflect the rates observed in practice.
The clinical development program for ERVEBO included clinical studies conducted in North America, Europe and Africa, in which 609 participants 12 months through 17 years of age and 18,007 participants 18 years of age and older received at least one dose of ERVEBO. The number of participants vaccinated with ERVEBO in double-blind, placebo-controlled trials was 2,913 and in open-label trials was 15,703.
In Study 1 (NCT02344407), conducted in Liberia (N=1,000), participants 18 years of age and older were randomized 1:1 to receive ERVEBO or saline placebo. Participants were assessed at Week 1 and Month 1 postvaccination for solicited local and systemic reactions. In a subset of participants (n=201), joint symptoms and signs were also solicited during a Week 2 visit. Memory aids were not used and postvaccination temperatures were measured only at study visits. Unsolicited adverse events were collected through Month 1 postvaccination. The median age of participants was 29 years, 63.6% were male and 100% were Black. Serious adverse events were monitored through 1-year postvaccination.
In Study 2 (NCT02503202), conducted in the United States, Canada and Spain (N=1,197), participants 18 through 65 years of age were randomized to receive ERVEBO (n=1,061) or saline placebo (n=133). Participants used a memory aid to record solicited local reactions from Days 1 to 5 postvaccination, and daily temperature measurements and solicited joint and skin events from Days 1 to 42 postvaccination. Unsolicited adverse reactions were collected through Day 42 postvaccination. The median age of participants was 42 years; 46.8% were male; 67.9% were White, 29.2% were Black or African American, 1.4% were Multi-racial, 0.8% were Asian, 0.4% were American Indian or Alaska Native, and 0.3% were Native Hawaiian or Pacific Islander; 14.5% were Hispanic or Latino. Serious adverse events were monitored through 6 months postvaccination, and a subset of participants (n=511) were monitored through 24 months postvaccination.
In Study 3 (Pan African Clinical Trials Registry, PACTR201503001057193), an open-label cluster-randomized study conducted in the Republic of Guinea, 5,643 participants 18 years of age and older received a dose of ERVEBO. The median age of vaccinated participants was 37 years, 68% were male and 100% were Black. Serious adverse events were monitored through 84 days postvaccination.
In Study 4 (NCT02378753), a randomized open-label study conducted in Sierra Leone, 7,998 participants 18 years of age and older received a dose of ERVEBO. The median age of participants was 31 years, 63% were male; 99.8% were Black and 0.2% collectively were Multi-racial, Asian or White. Serious adverse events were monitored through 180 days postvaccination.
In Study 5 (Pan African Clinical Trials Registry, PACTR201503001057193), an open-label safety and immunogenicity trial conducted in vaccinated frontline workers in the Republic of Guinea, implemented as Part B of Study 3, 2,016 participants 18 years of age and older received a dose of ERVEBO. The median age of vaccinated participants was 30 years, 75% were male and 100% were Black. Serious adverse events were monitored through 85 days postvaccination.
Study 6 (NCT02876328) is an ongoing, double-blind, randomized placebo-controlled study conducted in Liberia, Sierra Leone, Mali and the Republic of Guinea in which participants received ERVEBO or an investigational Ebola vaccine or placebo. A total of 155 participants 12 months through 2 years of age, 515 participants 3 through 11 years of age, 328 participants 12 through 17 years of age and 1,004 participants 18 years of age and older received a single dose of ERVEBO and saline placebo administered 56 days apart, or two doses of ERVEBO administered 56 days apart (not an approved dosing regimen), or two doses of saline placebo. Memory aids were not used. Participants were observed at the study site for 30 minutes after vaccination. Participants were assessed for solicited local and systemic reactions at study visits on day of vaccination (Day 0), Day 7, Day 14 and Day 28 after each vaccination. Postvaccination temperatures were obtained from participants 12 months through 17 years of age at daily contacts on Days 1 through 7, and on Day 14 and Day 28 after the first vaccination and Days 1 through 7 after the second vaccination. Postvaccination temperatures were obtained from participants 18 years of age and older at study visits. At each study visit, participants or their caregivers were queried about the occurrence of unsolicited adverse events. In this study, Grade 3 events were defined as symptoms causing inability to perform usual social and functional activities. Grade 4 events were defined as symptoms causing inability to perform basic self-care functions or medical or operative intervention indicated to prevent permanent impairment, persistent disability, or death. The median age of participants 18 years of age and older was 27 years and 54.6% were male, and the median age of participants 12 months through 17 years of age was 8 years and 54.7% were male. Serious adverse events were monitored through 12 months postvaccination.
Eight additional studies (NCT02269423, NCT02280408, NCT02374385, NCT02314923, NCT02287480, NCT02283099, NCT02296983) contributed to the assessment of serious adverse reactions.
Adverse Reactions in Participants 18 Years of Age and Older
Table 1 presents the proportion of participants reporting solicited adverse reactions in Study 1.
Table 1: Percentage of Participants 18 Years of Age and Older with Solicited Local and Systemic Adverse Reactions After Vaccination (Study 1) ERVEBO
%PLACEBO
%- * Adverse reactions were solicited at 30 minutes, Week 1 and Month 1 postvaccination.
- † Adverse reactions were solicited at Week 1 and Month 1 postvaccination.
- ‡ In a subset of participants (n=201), joint symptoms and signs were also solicited during a Week 2 visit.
Injection-site reactions* N=500 N=500 Injection site pain 34.0 11.2 Local reactions (redness/swelling) 1.8 0.8 Systemic adverse reactions† N=498 N=499 Headache 36.9 23.2 Feverishness 34.3 14.8 Muscle pain 32.5 22.8 Fatigue 18.5 13.4 Nausea 8.0 4.4 Joint pain/tenderness‡ 7.0 5.8 Rash 3.6 3.2 Abnormal sweating 3.2 2.6 Arthropathy (joint redness/warmth)‡ 0.6 0.2 Joint swelling‡ 0.4 0.4 Joint stiffness‡ 0.4 0.2 In Study 1, 56.4% of participants reported at least one of the solicited systemic adverse reactions listed in Table 1 within seven days after vaccination. With the exception of one participant who reported events of moderate intensity (causing greater than minimal interference with daily activity), all others reported events of mild intensity (causing no or minimal interference with daily activity).
Table 2 presents the proportion of participants 18 years of age and older reporting solicited adverse reactions in Study 2.
Table 2: Percentage of Participants 18 Years of Age and Older with Solicited Local and Systemic Adverse Reactions After Vaccination (Study 2) ERVEBO
%PLACEBO
%- * Adverse reactions were solicited Days 1 to 5 postvaccination.
- † Adverse reactions were solicited Day 1 through Day 42 postvaccination.
- ‡ Arthritis is a composite term that includes preferred terms of arthritis, monoarthritis, polyarthritis, osteoarthritis, joint swelling, or joint effusion.
- § Rash is a composite term that includes petechiae, purpura, rash, rash generalized, rash macular, rash papular and rash vesicular.
- ¶ Vesicular lesions include events reported as rash vesicular in the rash composite term and reported as blister.
Injection-site reactions* N=1051 N=133 Injection-site pain 69.5 12.8 Injection-site swelling 16.5 3.0 Injection-site redness 11.9 1.5 Systemic adverse reactions† N=1051 N=133 Joint pain 17.9 3.0 Arthritis (composite term)‡ 4.7 0.0 Rash (composite term)§ 3.8 1.5 Vesicular lesions¶ 1.5 0.0 In Study 2, 29 participants (2.8%) reported injection-site pain of severe intensity. Severe arthritis (arthritis and joint swelling) was reported by 8 participants (0.8%) and severe arthralgia was reported by 14 participants (1.3%). In this study, severe events were defined as incapacitating with inability to work or do usual activity.
Table 3 presents the proportion of participants 18 years of age and older reporting solicited adverse reactions in Study 6 within 28 days following administration of the first dose.
Table 3: Percentage of Participants 18 Years of Age and Older with Solicited Local and Systemic Adverse Reactions within 28 Days After the First Dose (Study 6) ERVEBO
%PLACEBO
%- * Adverse reactions were solicited postvaccination on day of vaccination (Day 0), Day 7, Day 14 and Day 28.
- † Feverishness was not defined by a specific temperature measurement.
- ‡ Includes: somnolence, reduced activity and fatigue.
Injection-site reactions* N=592 N=412 Injection-site pain 21.5 4.1 Injection-site swelling 3.7 2.9 Injection-site erythema 1.4 2.4 Systemic adverse reactions* N=592 N=412 Headache 55.1 43.4 Feverishness† 39.2 22.8 Myalgia 29.6 15.8 Somnolence‡ 25.5 13.6 Arthralgia 18.6 10.7 Chills 16.7 8.5 Decreased appetite 15.2 9.5 Abdominal pain 13.0 11.2 Nausea 9.5 6.3 Vomiting 4.4 1.2 Mouth ulceration 2.2 0.5 Abnormal sweating 1.4 1.0 Joint swelling 0.7 0.0 Most (>98%) reactions presented in Table 3 were mild to moderate in intensity.
Adverse Reactions in Participants 12 Months through 17 Years of Age
Table 4 presents the proportion of participants reporting solicited adverse reactions within 28 days following administration of the first dose in Study 6.
Table 4: Percentage of Participants 12 Months through 17 Years of Age with Solicited Local and Systemic Adverse Reactions within 28 Days After First Dose (Study 6) 12 Months through 2 Years of Age 3 Years through 11 Years of Age 12 Years through 17 Years of Age ERVEBO
%
N=95PLACEBO
%
N=60ERVEBO %
N=310PLACEBO
%
N=205ERVEBO %
N=203PLACEBO
%
N=123N/A: Not applicable because not assessed in this age group. - * Adverse reactions were solicited postvaccination on day of vaccination (Day 0), Day 1 through Day 7, Day 14 and Day 28.
- † Feverishness was not defined by a specific temperature measurement.
- ‡ Includes: somnolence, reduced activity and fatigue.
Injection-site reactions* Injection-site pain 26.3 8.3 39.4 8.8 52.2 17.1 Injection-site swelling 5.3 3.3 2.6 2.0 2.5 2.4 Injection-site erythema 1.1 3.3 0.3 0.5 0.5 0 Injection-site pruritus N/A N/A 6.5 0 2.5 0.8 Systemic adverse reactions* Feverishness† 83.2 66.7 64.8 37.1 48.3 27.6 Crying 30.5 6.7 3.2 2.4 N/A N/A Decreased appetite 27.4 15.0 23.9 13.2 20.7 14.6 Somnolence‡ 20.0 8.3 21.6 9.8 28.1 19.5 Diarrhea 18.9 16.7 2.9 4.4 3.9 4.1 Vomiting 16.8 10.0 11.0 8.8 3.9 3.3 Irritability 10.5 1.7 1.0 0.0 N/A N/A Screaming 9.5 1.7 0.6 0.5 N/A N/A Mouth ulceration 6.3 1.7 1.9 0.5 1.5 0 Chills 5.3 3.3 14.2 10.7 19.2 16.3 Headache 4.2 5.0 49.7 34.6 59.1 39.0 Abdominal pain 2.1 3.3 21.0 14.1 15.8 13.0 Abnormal sweating 2.1 3.3 1.3 2.0 4.9 0.8 Arthralgia N/A N/A 3.2 2.0 15.8 8.1 Dizziness N/A N/A 8.4 4.4 16.7 11.4 Myalgia N/A N/A 11.6 3.9 29.6 9.8 Nausea 0 1.7 8.4 3.4 8.4 8.1 Most (>98%) reactions presented in Table 4 were mild to moderate in intensity.
Table 5 presents the proportion of participants 12 months through 17 years of age reporting fever within 7 days following administration of the first dose in Study 6.
Table 5: Percentage of Participants 12 Months through 17 Years of Age with Fever Within 7 Days After the First Dose (Study 6)* Age Maximum Temperature
(Temporal)ERVEBO
%PLACEBO
%- * The use of antipyretic medication within 48 hours postvaccination was reported in 67% and 43% of participants 12 months through 2 years of age, 58% and 37% of participants 3 through 11 years of age, and 52% and 27% of participants 12 through 17 years of age for ERVEBO and placebo, respectively.
12 Months through 2 Years of Age
≥38.0°C to ≤38.4°C
>38.4°C to ≤38.9°C
>38.9°C to ≤40.0°C
>40.0°CN=95
3.2
3.2
0.0
0.0N=60
1.7
1.7
1.7
0.03 Years through 11 Years of Age
≥38.0°C to ≤38.4°C
>38.4°C to ≤38.9°C
>38.9°C to ≤40.0°C
>40.0°CN=310
4.2
1.0
1.3
0.0N=205
0.5
1.5
0.0
0.012 Years through 17 Years of Age
≥38.0°C to ≤38.4°C
>38.4°C to ≤38.9°C
>38.9°C to ≤40.0°C
>40.0°CN=203
2.5
2.5
2.5
0.0N=122
0.8
0.8
0.8
0.0Unsolicited Adverse Reactions
In Study 2, the unsolicited adverse reaction of chills was reported in 7.3% of ERVEBO recipients compared to 0% of placebo recipients. Paresthesia was reported by 1.4% of ERVEBO recipients compared to 0% of those who received placebo in this study.
Arthralgia and Arthritis
In an analysis across blinded, placebo-controlled studies (excluding Study 6), arthralgia was reported to occur in 7% to 40% of vaccine recipients. Arthralgia was generally reported in the first few days following vaccination, was of mild to moderate intensity, and resolved within one week after onset. Severe arthralgia, defined as preventing daily activity, was reported in up to 3% of participants.
In an analysis across blinded, placebo-controlled studies (excluding Study 6) in which participants received ERVEBO or a lower dose formulation, arthritis (including events of arthritis, joint effusion, joint swelling, osteoarthritis, monoarthritis or polyarthritis) was reported to occur in 0% to 24% of participants, with all but one study reporting arthritis in <5% of participants. Most occurrences of arthritis were reported within the first few weeks following vaccination, were of mild to moderate intensity, and resolved within several weeks after onset. In one study conducted in Switzerland (NCT02287480), 102 participants received ERVEBO or a lower dose formulation. In this study, arthritis was reported to occur in 24% of participants and severe arthritis, defined as preventing daily activity, in 12% of participants. Joint effusion samples were obtained from three participants and all three tested positive for vaccine virus RNA by RT-PCR. Of all 24 participants with arthritis in this study, six participants reported recurrent or prolonged joint symptoms lasting up to 2 years following vaccination, the longest follow-up period.
Rash
In an analysis across blinded, placebo-controlled studies (excluding Study 6), rash was reported to occur after administration of ERVEBO, with all but one study reporting rash in <9% of participants. In one study (NCT02287480), rash was reported to occur in 25% (n=4) of ERVEBO recipients and 7.7% (n=1) of placebo recipients. In this study, cutaneous vasculitis was reported in two participants who received a lower dose formulation, neither of whom had evidence of systemic vasculitis. Vesicular fluid and skin biopsy samples taken from some participants reporting rash have tested positive for vaccine virus RNA by RT-PCR.
Decreases in Lymphocytes and Neutrophils
White blood cell counts were assessed in 697 participants who received ERVEBO. Decreases in lymphocytes were reported in up to 85% of participants and decreases in neutrophils were reported in up to 43% of participants. No associated infections were reported.
Serious Adverse Reactions
In 18,616 ERVEBO recipients, two serious adverse reactions of pyrexia were reported as vaccine-related. In addition, two serious adverse reactions of anaphylaxis were reported as vaccine-related. These serious adverse reactions occurred in participants 18 years of age and older, and none were fatal.
-
7 DRUG INTERACTIONS
7.1 Interference with Laboratory Tests
Following vaccination with ERVEBO, individuals may test positive for anti-Ebola glycoprotein (GP) antibody and/or Ebola GP nucleic acid or antigens. GP-based testing may have limited diagnostic value during the period of vaccine viremia, in the presence of vaccine-derived Ebola GP, and following antibody response to the vaccine [see Pharmacokinetics (12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
There are no adequate and well-controlled studies of ERVEBO in pregnant women, and human data available from clinical trials with ERVEBO are insufficient to establish the presence or absence of vaccine-associated risk during pregnancy.
The decision to vaccinate a woman who is pregnant should consider the woman's risk of exposure to Zaire ebolavirus.
A developmental toxicity study has been performed in female rats administered a single human dose of ERVEBO on four occasions; twice prior to mating, once during gestation and once during lactation. This study revealed no evidence of harm to the fetus due to ERVEBO [see Animal Data below].
Clinical Considerations
Disease-associated Maternal and/or Embryo/Fetal Risk
Fetal and neonatal outcomes are universally poor among pregnant women infected with Zaire ebolavirus. The majority of such pregnancies end in miscarriage or stillbirth. In pregnancies where live birth does occur, neonates generally do not survive.
Data
Animal Data
In a developmental toxicity study, female rats received a single human dose of ERVEBO by intramuscular injection on four occasions: 28 days and 7 days prior to mating, gestation day 6 and lactation day 7. No adverse effects on pre-weaning development up to post-natal day 21 were observed. There were no vaccine-related fetal malformations or variations observed.
8.2 Lactation
Risk Summary
Human data are not available to assess the impact of ERVEBO on milk production, its presence in breast milk, or its effects on the breastfed child. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for ERVEBO and any potential adverse effects on the breastfed child from ERVEBO or from the underlying maternal condition. For preventive vaccines, the underlying condition is susceptibility to disease prevented by the vaccine.
8.4 Pediatric Use
The safety and effectiveness of ERVEBO in individuals 12 months through 17 years of age have been established [see Adverse Reactions (6.1) and Clinical Studies (14.2)]. The safety and effectiveness of ERVEBO in individuals younger than 12 months of age have not been established.
8.5 Geriatric Use
Across the clinical development program, the total number of participants ≥65 years of age who received at least one dose of ERVEBO was 554.
Clinical studies of ERVEBO did not include sufficient numbers of participants 65 years of age and older to determine whether they respond differently from younger participants.
-
11 DESCRIPTION
ERVEBO (Ebola Zaire Vaccine, Live) is a sterile suspension for intramuscular injection. ERVEBO is a live recombinant viral vaccine consisting of a vesicular stomatitis virus (VSV) backbone deleted for the VSV envelope glycoprotein and substituted with the envelope glycoprotein of the Zaire ebolavirus (Kikwit 1995 strain). The vaccine virus is grown in serum-free Vero cell cultures. The virus is harvested from the cell culture medium, purified, formulated with stabilizer solution, filled into vials and stored frozen. When thawed, ERVEBO is a colorless to slightly brownish-yellow liquid with no particulates visible.
Each 1 mL dose of ERVEBO contains a minimum of 72 million plaque forming units (pfu) of vaccine virus in a stabilizer solution containing 10 mM Tromethamine (Tris) and 2.5 mg/mL rice-derived recombinant human serum albumin. Each 1 mL dose may contain residual amounts of host cell DNA (≤10 ng) and benzonase (≤15 ng). The vaccine may contain trace amounts of rice protein. The product contains no preservatives.
The vaccine vial stopper is not made with natural rubber latex.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Immunization with ERVEBO results in an immune response and protection from disease caused by Zaire ebolavirus. The relative contributions of innate, humoral and cell-mediated immunity to protection from Zaire ebolavirus are unknown.
12.3 Pharmacokinetics
Viremia
Vaccine viremia was evaluated in 186 participants enrolled in seven clinical studies who were vaccinated with ERVEBO. Vaccine virus RNA was detected by RT-PCR in the plasma of most participants from Day 1 to Day 7 postvaccination with one participant having a positive plasma RT-PCR result 14 days after vaccination.
Shedding
Shedding of vaccine virus into the urine or saliva was evaluated in 359 participants enrolled in 8 clinical studies who were vaccinated with ERVEBO or lower dose formulations. Vaccine virus RNA was detected by RT-PCR in the urine or saliva of some participants at timepoints ranging from Day 1 through Day 14 postvaccination. In the 3 studies that assessed shedding at Day 28, no samples tested positive. In Study 6, 31.7% (19/60) of participants 12 months through 17 years of age enrolled in a substudy shed vaccine virus in saliva following vaccination. Viral shedding was greatest on Day 7 and declined thereafter, with no shedding detected after Day 28.
Vaccine virus RNA was detected by RT-PCR in vesicular fluid samples from some participants. In one participant, a sample collected 20 days after vaccination tested positive for vaccine virus RNA by RT-PCR.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
ERVEBO has not been evaluated for the potential to cause carcinogenicity, genotoxicity or impairment of male fertility. ERVEBO administered to female rats had no effects on fertility [see Use in Specific Populations (8.1)].
-
14 CLINICAL STUDIES
14.1 Clinical Efficacy
Clinical efficacy of ERVEBO was assessed in Study 3.
Study 3 (Ring vaccination study) was an open-label, randomized cluster (ring) vaccination study conducted in the Republic of Guinea during the 2014 outbreak. Each cluster was composed of contacts and contacts of contacts of individuals with laboratory-confirmed Ebola virus disease (EVD). Clusters were randomized to receive either an "immediate" vaccination or a 21-day "delayed" vaccination. In the primary efficacy analysis, 3,537 participants ≥18 years of age were considered contacts and contacts of contacts of an index case with laboratory-confirmed EVD. Of these, 2,108 were included in 51 immediate vaccination clusters, and 1,429 were included in 46 delayed vaccination clusters.
The median age of participants in the primary efficacy analysis was 40 years. The majority were male, comprising 70.4% and 70.3% in the randomized immediate and delayed clusters, respectively.
In the primary efficacy analysis, the number of cases of laboratory-confirmed EVD in participants vaccinated in immediate vaccination clusters was compared to the number of cases in participants in delayed vaccination clusters. Cases of EVD that occurred between Day 10 and Day 31 post-randomization of the cluster were included in the analysis. Vaccine efficacy was 100% (95% CI: 63.5% to 100%); no cases of confirmed EVD were observed in the immediate vaccination clusters, and 10 confirmed cases of EVD were observed in a total of 4 delayed vaccination clusters between Day 10 and Day 31 post-randomization.
14.2 Clinical Immunogenicity
A measure of the immune response that confers protection against EVD is unknown.
Clinical Immunogenicity in Individuals 18 Years of Age and Older Across Geographic Areas
Four studies assessed antibody responses to ERVEBO in participants 18 years of age and older (Study 1, Study 2, Study 4 and Study 5), including 477 participants in Liberia, 506 participants in Sierra Leone, 915 participants in the US, Canada, and Spain (n=865 US participants) and 1,217 participants in the Republic of Guinea. Zaire ebolavirus (Kikwit) GP-specific immunoglobulin G (IgG) was detected by enzyme linked immunosorbent assay (GP-ELISA). Vaccine virus neutralizing antibody was detected by a plaque reduction neutralization test (PRNT).
Antibody responses among participants in the study conducted in the US, Canada, and Spain (Study 2) were similar to those among participants in the studies conducted in Liberia (Study 1), Sierra Leone (Study 4) and the Republic of Guinea (Study 5).
Clinical Immunogenicity in Individuals 12 Months through 17 Years of Age
A fifth study (Study 6) conducted in Liberia, Sierra Leone, Mali and the Republic of Guinea assessed antibody geometric mean titers (GMT) to ERVEBO in 386 participants 12 months through 17 years of age compared to 386 participants 18 years of age and older. Zaire ebolavirus (Kikwit) GP-specific immunoglobulin G (IgG) was detected by enzyme linked immunosorbent assay (GP-ELISA). GMTs at Day 28 after vaccination with ERVEBO in participants 12 months through 17 years of age were non-inferior to those in participants 18 years of age and older (see Table 6).
Table 6: Non-inferiority Analysis of Pooled Geometric Mean Titers at Day 28 for the GP-ELISA (Study 6) Individuals 12 Months through
17 Years of Age
n=499Individuals 18 Years of Age
and Older
n=519GMT ratio* (95% CI) The Per-Protocol Immunogenicity Population was the primary population for the immunogenicity analyses in Study 6 and includes all vaccinated participants with serology data who were compliant with the protocol and had a serum sample collected within an acceptable day range.
n=Number of participants contributing to the analysis.
CI=Confidence interval; GMT=geometric mean titer; GP-ELISA=glycoprotein enzyme-linked immunosorbent assay.
Study 6 used gamma irradiation of specimens to reduce risk of wild-type Ebola virus infection of laboratory workers.- * GMT (individuals 12 months through 17 years of age) / GMT (individuals 18 years of age and older); Non-inferiority is declared if the lower bound of the 2-sided 95% CI for the GMT ratio is greater than 0.5.
GMT (95% CI) GMT (95% CI) 1.42 (1.24, 1.62) 1748.8 (1585.6, 1928.7) 1234.4 (1132.5, 1345.4) -
16 HOW SUPPLIED/STORAGE AND HANDLING
Carton of ten 1 mL single-dose vials. NDC: 0006-4293-02
Store frozen at -80°C to -60°C (-112°F to -76°F). Store in the original carton to protect from light.
Do not thaw the vial in a refrigerator. Thaw the vial at room temperature until no visible ice is present. Use the vaccine immediately after thawing. If not used immediately, a thawed vial can be stored refrigerated at 2°C to 8°C (35.6°F to 46.4°F) for a total time of no more than 2 weeks and at room temperature (up to 25°C; 77°F) for a total time of no more than 4 hours. Protect from light. Do not re-freeze thawed vaccine.
-
17 PATIENT COUNSELING INFORMATION
Advise the vaccine recipients, parents or guardians to read the FDA-approved patient labeling (Patient Information).
Advise vaccine recipients, parents or guardians of the following:
- ERVEBO has not been demonstrated to provide protection against disease caused by viruses other than Zaire ebolavirus. After vaccination with ERVEBO, individuals at risk should continue to protect themselves from exposure to Zaire ebolavirus.
- ERVEBO may not protect all vaccinated individuals.
- Transmission of vaccine virus is a theoretical possibility. Vaccine virus RNA has been detected in blood, saliva, or urine for up to 14 days after vaccination. The duration of shedding is not known; however, samples taken 28 days after vaccination tested negative. Vaccine virus RNA has been detected in fluid from skin vesicles that appeared after vaccination.
Instruct vaccine recipients, parents or guardians to:
- Report any adverse reactions to their health care provider.
- Seek immediate medical attention if any signs or symptoms of a hypersensitivity reaction occur after vaccination [see Contraindications (4)].
-
SPL UNCLASSIFIED SECTION
Manufactured by: Merck Sharp & Dohme LLC
Rahway, NJ 07065, USAFor patent information: www.msd.com/research/patent
Copyright © 2019-2023 Merck & Co., Inc., Rahway, NJ, USA, and its affiliates.
All rights reserved.uspi-v920-i-2307r003
-
PATIENT PACKAGE INSERT
Patient Information about
ERVEBO® (pronounced "er-VEE-boh")
(Ebola Zaire Vaccine, Live)Before you or your child get ERVEBO®, read this document and be sure you understand all of the information. Keep this document. You may need to read it again. If you have questions or side effects, ask the health care provider. This information does not take the place of talking about ERVEBO with the health care provider.
You or your child may be advised to get this vaccine in an emergency situation.
What is ERVEBO?
- ERVEBO is a vaccine to prevent disease caused by Zaire ebolavirus in individuals who are 12 months of age and older.
- This vaccine is given to help protect you or your child from getting Ebola virus disease caused by one type of Ebola virus (Zaire ebolavirus). This vaccine will not protect against disease caused by other types of Ebola virus.
- You or your child cannot get Ebola virus disease from ERVEBO.
- After getting ERVEBO, you or your child should continue to protect against exposure to Ebola virus.
- ERVEBO might not protect everyone who gets the vaccine.
What should I tell the health care provider before getting ERVEBO?
You should tell the health care provider if you or your child:
- have ever had an allergic reaction to a vaccine or medicine
- have ever had an allergic reaction to ERVEBO, rice protein or any of the ingredients in this vaccine
- are pregnant or breastfeeding
- think you or your child may be pregnant or are planning to have a baby
- have a weakened immune system or take medicines or treatments that might weaken the immune system
- have close contact with anyone who has a weakened immune system
How will I get ERVEBO?
ERVEBO is given as an injection.
What are the side effects of ERVEBO?
Serious allergic reactions have been observed after administration of ERVEBO. Tell the health care provider promptly about any unusual or severe symptoms that develop after getting this vaccine. Get medical care right away if you or your child have signs of an allergic reaction, which may include:
- wheezing or trouble breathing
- swelling of the face, lips, tongue, or other parts of the body
- generalized itching, redness, flushing or itchy bumps on the skin
Other side effects in individuals 18 years of age and older:
- Pain, swelling, or redness at the injection site
- Fever
- Feeling tired
- Joint pain
- Muscle aches
- Headache
- Stomach pain
- Chills
- Abnormal sweating
- Nausea
- Skin rash (including blisters)
- Joint swelling
- Eating less than usual
- Mouth sores
- Vomiting
Certain white blood cell counts can become lower than normal after vaccination but are not associated with illness and go back to normal.
Most side effects go away within a few days. Joint pain and swelling may last for weeks, months, or years in some people. In some people, joint pain and swelling may come back after initially going away.
Side effects in individuals 12 months through 17 years of age:
- Pain, itching, swelling, or redness at the injection site
- Fever
- Headache
- Feeling tired
- Eating less than usual
- Muscle aches
- Crying
- Feeling dizzy
- Mouth sore
- Abdominal pain
- Vomiting
- Chills
- Diarrhea
- Joint pain
- Irritability
- Screaming
- Abnormal sweating
- Nausea
If you or your child have any side effect that bothers you or your child or does not go away, tell the doctor, pharmacist or nurse.
There may be other side effects that are not listed here.
Talk to the doctor, pharmacist or nurse with questions or concerns about possible side effects.
You may also report any side effects to Merck Sharp & Dohme LLC at 1-877-888-4231 or directly to Vaccine Adverse Event Reporting System (VAERS). The VAERS toll-free number is 1-800-822-7967 or report online to www.vaers.hhs.gov .Other information
- ERVEBO may cause you or your child to have a positive test for Ebola, even if you or your child don't have the disease. Tell the health care provider that you or your child received ERVEBO. The health care provider might need to do a different test.
What is in ERVEBO?
Active ingredient: live Vesicular Stomatitis Virus in which the surface protein has been replaced with that of Zaire ebolavirus.
Inactive ingredients: recombinant human serum albumin, tromethamine (Tris) buffer.
This vaccine contains a trace amount of rice protein.
What if I have other questions?
- To learn more about ERVEBO, talk to the health care provider.
- Keep this information about ERVEBO. You might want to look at it again.
-
SPL UNCLASSIFIED SECTION
Manufactured by: Merck Sharp & Dohme LLC
Rahway, NJ 07065, USAFor patent information: www.msd.com/research/patent
Copyright © 2019-2025 Merck & Co., Inc., Rahway, NJ, USA, and its affiliates.
All rights reserved.
usppi-v920-i-2501r003
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: 01/2025
-
PRINCIPAL DISPLAY PANEL
NDC: 0006-4293-02
10 Single-dose 1 mL Vials
10 flacons de 1 ml à dose uniqueSTORE AT / CONSERVER ENTRE
-80°C to -60°CEbola Zaire Vaccine, Live
Vaccin Ebola Zaïre, Vivant
ERVEBO®For Intramuscular Use Only / Pour l'utilisation Intramusculaire Uniquement
Vaccine virus titer ≥ 7.2x107 pfu/mL
Concentration du virus dans le vaccin: ≥ 7.2x107 UFP (Unités Formant Plage)/mlDiscard unused portion / Jeter toute portion inutilisée.
Rx only
Uniquement par prescription.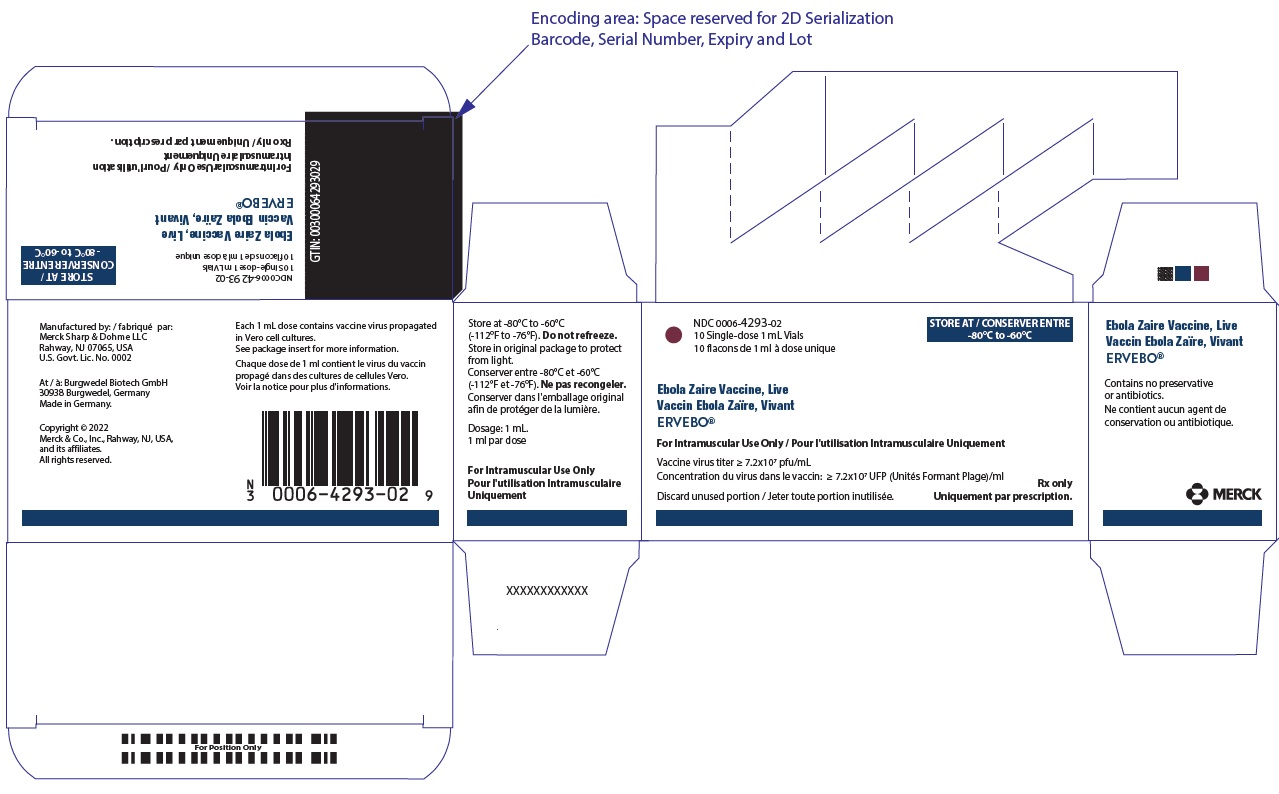
-
INGREDIENTS AND APPEARANCE
ERVEBO
zaire ebolavirus (strain kikwit-95) envelope glycoprotein injection, solutionProduct Information Product Type VACCINE Item Code (Source) NDC: 0006-4293 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZAIRE EBOLAVIRUS (STRAIN KIKWIT-95) ENVELOPE GLYCOPROTEIN (UNII: XH5V2SQ5FI) (ZAIRE EBOLAVIRUS (STRAIN KIKWIT-95) ENVELOPE GLYCOPROTEIN - UNII:XH5V2SQ5FI) ZAIRE EBOLAVIRUS (STRAIN KIKWIT-95) ENVELOPE GLYCOPROTEIN 72000000 [PFU] in 1 mL Inactive Ingredients Ingredient Name Strength Tromethamine (UNII: 023C2WHX2V) 0.01 mmol in 1 mL ALBUMIN HUMAN (UNII: ZIF514RVZR) 2.5 mg in 1 mL WATER (UNII: 059QF0KO0R) Product Characteristics Color WHITE (off-white) , YELLOW (pale yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0006-4293-02 10 in 1 CARTON 1 NDC: 0006-4293-01 1.0 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125690 12/19/2019 Labeler - Merck Sharp & Dohme LLC (118446553)
Trademark Results [ERVEBO]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ERVEBO 88737072 not registered Live/Pending |
Merck Sharp & Dohme Corp. 2019-12-23 |
 ERVEBO 88737069 not registered Live/Pending |
Merck Sharp & Dohme Corp. 2019-12-23 |
 ERVEBO 86808776 5503482 Live/Registered |
Merck Sharp & Dohme Corp. 2015-11-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.