HurriCaine Gel LV Syringe - Watermelon
HurriCaine by
Drug Labeling and Warnings
HurriCaine by is a Otc medication manufactured, distributed, or labeled by Beutlich Pharmaceuticals, LLC, Dental Technologies, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
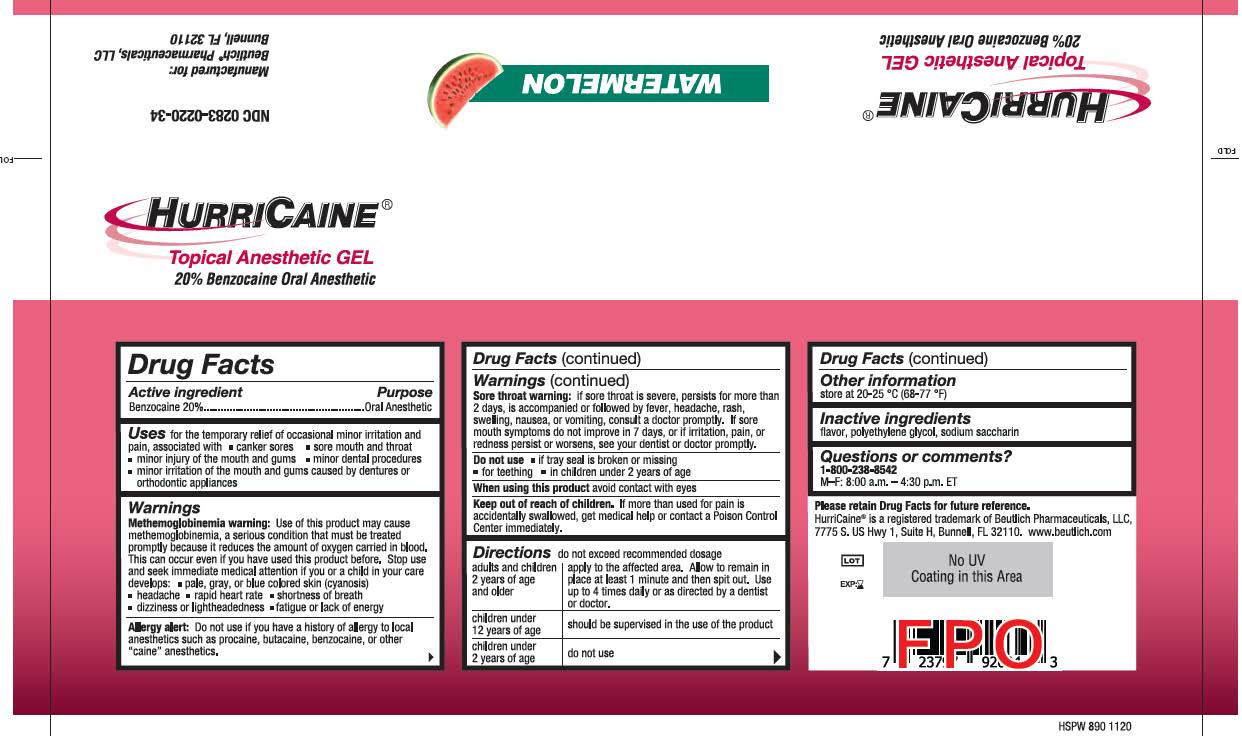
HURRICAINE TOPICAL ANESTHETIC- benzocaine gel
Beutlich Pharmaceuticals, LLC
----------
HurriCaine Gel LV Syringe - Watermelon
Uses
for the temporary relief of occasional minor irritation and pain, associated with:
- canker sores
- sore mouth and throat
- minor injury of the mouth and gums
- minor dental procedures
- minor irritation of the mouth and gums caused by dentures or orthodontic appliances
Warnings
Methemoglobinemia warning: Use of this product may cause methemoglobinemia, a serious condition that must be treated promptly because it reduces the amount of oxygen carried in blood. This can occur even if you have used this product before. Stop use and seek immediate medical attention if you or a child in your care develops:
pale, gray, or blue colored skin (cyanosis)
headache
rapid heart rate
shortness of breath
dizziness or lightheadedness
fatigue or lack of energy
Allergy alert: Do not use if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other "caine" anesthetics.
Sore throat warning: if sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, swelling, nausea, or vomiting, consult a doctor promptly. If sore mouth symptoms do not improve in 7 days, or irritation, pain, or redness persists or worsens, see your dentist or doctor promptly.
Do not use
Do not use:
- if the tray seal is broken or missing
- if cap is missing or damaged
- for teething
- in children under 2 years of age
Keep out of reach of children.
If more than used for pain is accidentally swallowed, get medical help or contact a Poison Control Center immediately.
Directions
Directions:
Do not exceed recommended dosage.
- adults and children 2 years of age and older: apply to the affected area. Allow to remain in place at least 1 minute and then spit out. Use up to 4 times daily or as directed by a dentist or doctor.
- children under 12 years of age: should be supervised in the use of the product
- children under 2 years of age: do not use
| HURRICAINE
TOPICAL ANESTHETIC
benzocaine gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Beutlich Pharmaceuticals, LLC (005209325) |
| Registrant - Beutlich Pharmaceuticals, LLC (005209325) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Dental Technologies, Inc. | 148312838 | manufacture(0283-0220) | |
Trademark Results [HurriCaine]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 HURRICAINE 72442584 0971198 Live/Registered |
BEUTLICH, INC. 1972-11-30 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.