NAYZILAM- midazolam spray
Nayzilam by
Drug Labeling and Warnings
Nayzilam by is a Prescription medication manufactured, distributed, or labeled by UCB, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use NAYZILAM® safely and effectively. See full prescribing information for NAYZILAM®.
NAYZILAM® (midazolam) nasal spray, CIV
Initial U.S. Approval: 1985INDICATIONS AND USAGE
NAYZILAM is a benzodiazepine indicated for the acute treatment of intermittent, stereotypic episodes of frequent seizure activity (i.e., seizure clusters, acute repetitive seizures) that are distinct from a patient's usual seizure pattern in patients with epilepsy 12 years of age and older. (1)
DOSAGE AND ADMINISTRATION
Administer NAYZILAM by the nasal route only. (2.2)
Initial Dose: Administer one spray (5 mg dose) into one nostril. (2.2)
Second Dose: One additional spray (5 mg dose) into the opposite nostril may be administered after 10 minutes if the patient has not responded to the initial dose. (2.2)
Maximum Dosage and Treatment Frequency: Do not use more than 2 doses of NAYZILAM to treat a seizure cluster. It is recommended that NAYZILAM be used to treat no more than one episode every three days and treat no more than five episodes per month. (2.2)
DOSAGE FORMS AND STRENGTHS
Single-dose nasal spray unit containing 5 mg midazolam per 0.1 mL solution. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- CNS Depression From Concomitant Use With Other CNS Depressants or Moderate or Strong CYP3A4 Inhibitors: May cause an increased CNS-depressant effect when used with alcohol or other CNS depressants. Concomitant use with moderate or strong CYP3A4 inhibitors may result in prolonged sedation because of a decrease in plasma clearance of midazolam. (5.3, 7.3)
- Suicidal Behavior and Ideation: Antiepileptic drugs increase the risk of suicidal ideation and behavior. (5.4)
- Impaired Cognitive Function: Midazolam is associated with a high incidence of partial or complete impairment of recall for the next several hours. (5.5)
ADVERSE REACTIONS
The most common adverse reactions (≥5% in any NAYZILAM treatment group) were somnolence, headache, nasal discomfort, throat irritation, and rhinorrhea (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact UCB, Inc. at 1-844-599-2273 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- CYP3A4 Inhibitors: Avoid co-administration of NAYZILAM with moderate or strong CYP3A4 inhibitors. NAYZILAM should be used with caution when co-administered with mild CYP3A4 inhibitors. (7.1)
- Opioids: Risk of respiratory depression is increased. (7.2)
- Other CNS Depressants: May increase the risks of hypoventilation, airway obstruction, desaturation, or apnea and may contribute to profound and/or prolonged drug effect. (7.3)
USE IN SPECIFIC POPULATIONS
- Pregnancy: Based on animal data, may cause fetal harm. (8.1)
- Lactation: Midazolam is excreted in human milk. Caution should be exercised when NAYZILAM is administered to a nursing woman. (8.2)
- Renal Impairment: Patients with renal impairment may have longer elimination half-lives for midazolam and its metabolites which may result in prolonged exposure. (8.6)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 5/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Instructions Prior to Dosing
2.2 Dosage Information
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risks from Concomitant Use with Opioids
5.2 Risks of Cardiorespiratory Adverse Reactions
5.3 Central Nervous System Depression from Concomitant Use with Other Central Nervous System Depressants, or Moderate or Strong CYP3A4 Inhibitors
5.4 Suicidal Behavior and Ideation
5.5 Impaired Cognitive Function
5.6 Glaucoma
5.7 Other Adverse Reactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Congestive Heart Failure
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
9.4 Chronic Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED / STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS
Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death [see Warnings and Precautions (5.1), Drug Interactions (7.2)].
- Reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate.
- Limit dosages and durations to the minimum required.
- Follow patients for signs and symptoms of respiratory depression and sedation.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Instructions Prior to Dosing
NAYZILAM prescribers should consider the following prior to initiation of treatment:
For patients at increased risk of respiratory depression from benzodiazepines, administration of NAYZILAM under healthcare professional supervision should be considered prior to treatment with NAYZILAM; this administration may be performed in the absence of a seizure episode [see Warnings and Precautions (5.2)].
Prior to treatment, the healthcare professional should instruct the individual administering NAYZILAM on how to identify seizure clusters and use the product appropriately [see Patient Counseling Information: Administration Information (17)]. Patients and caregivers should be counseled to read carefully the "Instructions for Use" for complete directions on how to properly administer NAYZILAM.
2.2 Dosage Information
Administer NAYZILAM by the nasal route only.
Second Dose (if needed): One additional spray (5 mg dose) into the opposite nostril may be administered after 10 minutes if the patient has not responded to the initial dose.
A second dose of NAYZILAM should not be administered if the patient has trouble breathing or if there is excessive sedation that is uncharacteristic of the patient during a seizure cluster episode [see Warnings and Precautions (5.2)].
Maximum Dosage and Treatment Frequency: Do not use more than 2 doses of NAYZILAM to treat a single episode.
It is recommended that NAYZILAM be used to treat no more than one episode every three days and no more than 5 episodes per month [see Drug Abuse and Dependence (9.4)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
NAYZILAM is contraindicated in patients with:
- Known hypersensitivity to midazolam.
- Acute narrow-angle glaucoma [see Warnings and Precautions (5.6)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Risks from Concomitant Use with Opioids
Concomitant use of benzodiazepines, including NAYZILAM, and opioids may result in profound sedation, respiratory depression, coma, and death. Because of these risks, reserve concomitant prescribing of benzodiazepines and opioids for use in patients for whom alternative treatment options are inadequate.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioids alone. If a decision is made to prescribe NAYZILAM concomitantly with opioids, prescribe the lowest effective dosages and minimum durations of concomitant use, and follow patients closely for signs and symptoms of respiratory depression and sedation. Advise both patients and caregivers about the risks of respiratory depression and sedation when NAYZILAM is used with opioids [see Drug Interactions (7.2)].
5.2 Risks of Cardiorespiratory Adverse Reactions
Serious cardiorespiratory adverse reactions have occurred after administration of midazolam. These have included respiratory depression, airway obstruction, oxygen desaturation, apnea, respiratory arrest and/or cardiac arrest, sometimes resulting in death or permanent neurologic injury. There have also been rare reports of hypotensive episodes requiring treatment during or after diagnostic or surgical manipulations, particularly in patients with hemodynamic instability. Hypotension occurs more frequently in patients premedicated with a narcotic. The danger of hypoventilation, airway obstruction, or apnea is greater in elderly patients and those with chronic disease states or decreased pulmonary reserve [see Use in Specific Populations (8.5)]; patients with chronic obstructive pulmonary disease are highly sensitive to the respiratory depressant effect of midazolam.
Respiratory depression was observed with the administration of NAYZILAM during clinical trials [see Adverse Reactions (6.1)]. Cardiac or respiratory arrest caused by NAYZILAM was not reported during clinical trials.
5.3 Central Nervous System Depression from Concomitant Use with Other Central Nervous System Depressants, or Moderate or Strong CYP3A4 Inhibitors
Drug products containing midazolam, including NAYZILAM, have a central nervous system (CNS) depressant effect.
Risks from Concomitant Use with Other CNS Depressants
The potential for an increased CNS-depressant effect from concomitant use with alcohol or other CNS depressants (e.g., opioids) must be considered by the prescribing physician, and appropriate recommendations made to the patient and/or caregiver [see Warnings and Precautions (5.1) and Drug Abuse and Dependence (9.3)].
Concomitant use of barbiturates, alcohol, or other CNS depressants may increase the risk of hypoventilation, airway obstruction, desaturation, or apnea and may contribute to profound and/or prolonged drug effect [see Drug Interactions (7.3)].
Risks from Concomitant Use with Moderate or Strong CYP3A4 Inhibitors
There is a potential for prolonged sedation from concomitant use with moderate or strong CYP3A4 enzyme inhibitors because of much higher midazolam exposures [see Drug Interactions (7.2) and Clinical Pharmacology (12.2)].
5.4 Suicidal Behavior and Ideation
Antiepileptic drugs (AEDs), including NAYZILAM, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted Relative Risk 1.8, 95% CI:1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence rate of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43%, compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug-treated patients in the trials and none in placebo-treated patients, but the number is too small to allow any conclusion about drug effect on suicide.
The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting drug treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed. The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs used for any indication. The risk did not vary substantially by age (5-100 years) in the clinical trials analyzed. Table 1 shows absolute and relative risk by indication for all evaluated AEDs.
Table 1. Risk by Indication for Antiepileptic Drugs in the Pooled Analysis Indication Placebo Patients with Events/1000 Patients Drug Patients with Events per 1000 Patients Relative Risk: Incidence of Drug Events in Drug Patients /Incidence in Placebo Patients Risk Difference: Additional Drug Patients with Events per 1000 Patients Epilepsy 1.0 3.4 3.5 2.4 Psychiatric 5.7 8.5 1.5 2.9 Other 1.0 1.8 1.9 0.9 Total 2.4 4.3 1.8 1.9 The relative risk for suicidal thoughts or behavior was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.
Anyone considering prescribing midazolam or any other AED must balance the risk of suicidal thoughts or behaviors with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
5.5 Impaired Cognitive Function
Midazolam, including NAYZILAM, is associated with a high incidence of partial or complete impairment of recall for several hours following an administered dose. Gross tests of recovery from the effects of midazolam cannot be relied upon to predict reaction time under stress. It is recommended that no patient operate hazardous machinery or a motor vehicle until the effects of the drug, such as drowsiness, have subsided, and as their medical condition permits. For pediatric patients, particular care should be taken to ensure safe ambulation.
5.6 Glaucoma
Benzodiazepines, including NAYZILAM, can increase intraocular pressure in patients with glaucoma. Measurements of intraocular pressure in patients without eye disease show a moderate lowering following induction with midazolam. NAYZILAM may be used in patients with open-angle glaucoma only if they are receiving appropriate therapy. Patients with open-angle glaucoma may need to have their ophthalmologic status evaluated following treatment with NAYZILAM. NAYZILAM is contraindicated in patients with narrow-angle glaucoma.
5.7 Other Adverse Reactions
When midazolam was used for sedation, reactions such as agitation, involuntary movements (including tonic/clonic movements and muscle tremor), hyperactivity, and combativeness have been reported. These reactions may be caused by inadequate or excessive dosing or improper administration of midazolam; however, consideration should be given to the possibility of cerebral hypoxia or true paradoxical reactions.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in more detail in other sections of the labeling:
- Risks from Concomitant Use with Opioids [see Warnings and Precautions (5.1)]
- Risks of Cardiorespiratory Adverse Reactions [see Warnings and Precautions (5.2)]
- CNS Depression from Concomitant Use with Other CNS Depressants or Moderate or Strong CYP3A4 Inhibitors [see Warnings and Precautions (5.3)]
- Suicidal Behavior and Ideation [see Warnings and Precautions (5.4)]
- Impaired Cognitive Function [see Warnings and Precautions (5.5)]
- Glaucoma [see Warnings and Precautions (5.6)]
- Other Adverse Reactions [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
NAYZILAM was studied for the outpatient treatment of a single seizure cluster in 292 adult and adolescent patients with epilepsy (Study 1) [see Clinical Studies (14)]. The study was conducted in two phases; an open-label Test Dose Phase followed by a double-blind, placebo-controlled, Comparative Phase. The mean age of patients enrolled in the Comparative Phase (N=201) was 33 years, 51% were female, and 95% were White.
Table 2 lists the adverse reactions occurring in 2% or more of the NAYZILAM-treated patients and at a rate greater than the placebo-treated patients in the Comparative Phase of Study 1.
Table 2: Adverse Reactions* that Occurred in ≥2% of Patients (Any NAYZILAM) and Greater than Placebo in the Comparative Phase of Study 1 Body System/Adverse Reaction Placebo NAYZILAM† NAYZILAM
5 mgPlacebo + NAYZILAM
5 mgNAYZILAM
5 mg + 5 mgAny NAYZILAM Treatment Group N = 26
%N = 91
%N = 41
%N = 43
%N = 175
%- * Adverse reactions that occurred within 2 days after NAYZILAM administration are included
- † Patients in Study 1 were permitted to take a second, open-label dose of NAYZILAM 5 mg between 10 minutes and 6 hours following the initial blinded dose of NAYZILAM 5 mg or placebo if they experience seizure recurrence or an incomplete resolution of the episode. The Placebo + NAYZILAM 5 mg and NAYZILAM 5 mg + 5 mg columns represent patients who received a second dose of NAYZILAM 5 mg and received a blinded initial dose of placebo or NAYZILAM 5 mg, respectively.
Nervous System Somnolence 4 10 10 9 10 Headache 0 7 0 2 4 Dysarthria 0 2 2 2 2 Application Site Nasal Discomfort 8 5 7 16 9 Throat Irritation 0 2 2 7 3 Rhinorrhea 0 3 0 5 3 Product Taste Abnormal 0 4 0 0 2 Eye Disorders Lacrimation Increased 0 1 2 2 2 For patients who experienced a decrease in peripheral oxygen saturation in the Test Dose Phase of Study 1, the decreases were generally transitory. Two patients (one with a history of sleep apnea and one with intercurrent seizure) with decreases in peripheral oxygen saturation in the Test Dose Phase required therapeutic supplemental oxygen.
-
7 DRUG INTERACTIONS
Table 3: Clinically Significant Drug Interactions With NAYZILAM Clinical Impact: Concomitant use of CYP3A4 inhibitors may result in prolonged sedation because of a decrease in plasma clearance of midazolam. Intervention: Avoid co-administration of NAYZILAM with moderate or strong CYP3A4 inhibitors. NAYZILAM should be used with caution when co-administered with mild CYP3A4 inhibitors. Examples: Moderate CYP3A4 inhibitors: erythromycin, diltiazem, verapamil
Strong CYP3A4 inhibitors: ketoconazole, itraconazole, clarithromycinClinical Impact: The concomitant use of benzodiazepines and opioids increases the risk of respiratory depression because of actions at different receptor sites in the CNS that control respiration. Benzodiazepines interact at GABAA sites and opioids interact primarily at mu receptors. When benzodiazepines and opioids are combined, the potential for benzodiazepines to significantly worsen opioid-related respiratory depression exists. Intervention: Reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required [see Warnings and Precautions (5.1)]. Examples: Morphine, hydrocodone, oxymorphone, codeine, fentanyl Clinical Impact: Concomitant use of barbiturates, alcohol, or other CNS depressants may increase the risk of hypoventilation, airway obstruction, desaturation, or apnea and may contribute to profound and/or prolonged drug effect. Intervention: Reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required [see Warnings and Precautions (5.3)]. Examples: Other benzodiazepines and sedatives/hypnotics, anxiolytics, tranquilizers, muscle relaxants, general anesthetics, antipsychotics, opioids, alcohol. -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to antiepileptic drugs (AEDs), such as NAYZILAM, during pregnancy. Encourage women who are taking NAYZILAM during pregnancy to enroll in the North American Antiepileptic Drug (NAAED) pregnancy registry by calling 1-888-233-2334 or visiting http://www.aedpregnancyregistry.org/.
Risk Summary
There are no adequate and well-controlled studies of NAYZILAM in pregnant women.
Available data suggest that the class of benzodiazepines is not associated with marked increases in risk for congenital anomalies. Although some early epidemiological studies suggested a relationship between benzodiazepine drug use in pregnancy and congenital anomalies such as cleft lip and or palate, these studies had considerable limitations. More recently completed studies of benzodiazepine use in pregnancy have not consistently documented elevated risks for specific congenital anomalies. There is insufficient evidence to assess the effect of exposure to benzodiazepines during pregnancy on neurodevelopment.
There are clinical considerations regarding exposure to benzodiazepines during the second and third trimesters of pregnancy or immediately prior to or during childbirth. These risks include decreased fetal movement and/or fetal heart rate variability, "floppy infant syndrome," dependence, and withdrawal (see Clinical Considerations and Human Data).
Administration of midazolam to rats and rabbits during the period of organogenesis or to rats during late pregnancy and throughout lactation at doses greater than those used clinically did not result in any apparent adverse effects on development (see Animal Data). However, published data for midazolam and other benzodiazepines suggest the possibility of neuronal cell death and long-term effects on neurobehavioral and immunological function in animals following prenatal or early postnatal exposure at clinically relevant doses. NAYZILAM should be used during pregnancy only if the potential benefit to the mother justifies the potential risk to the fetus. Advise a pregnant woman and women of childbearing age of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively. The background risk of major birth defects and miscarriage for the indicated population is unknown.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Infants born to mothers who have taken benzodiazepines during the later stages of pregnancy can develop dependence, and subsequently withdrawal, during the postnatal period. Clinical manifestations of withdrawal or neonatal abstinence syndrome may include hypertonia, hyperreflexia, hypoventilation, irritability, tremors, diarrhea, and vomiting. These complications can appear shortly after delivery to 3 weeks after birth and persist from hours to several months depending on the degree of dependence and the pharmacokinetic profile of the benzodiazepine. Symptoms may be mild and transient or severe. Standard management for neonatal withdrawal syndrome has not yet been defined. Observe newborns who are exposed to NAYZILAM in utero during the later stages of pregnancy for symptoms of withdrawal and manage accordingly.
Labor and Delivery
Administration of benzodiazepines immediately prior to or during childbirth can result in a floppy infant syndrome, which is characterized by lethargy, hypothermia, hypotonia, respiratory depression, and difficulty feeding. Floppy infant syndrome occurs mainly within the first hours after birth and may last up to 14 days. Observe exposed newborns for these symptoms and manage accordingly.
Data
Human Data
Congenital Anomalies
Although there are no adequate and well-controlled studies of NAYZILAM in pregnant women, there is information about benzodiazepines as a class. Dolovich et al. published a meta-analysis of 23 studies that examined the effects of benzodiazepine exposure during the first trimester of pregnancy. Eleven of the 23 studies included in the meta-analysis considered the use of chlordiazepoxide and diazepam and not other benzodiazepines. The authors considered case-control and cohort studies separately. The data from the cohort studies did not suggest an increased risk for major malformations (OR 0.90; 95% CI 0.61—1.35) or for oral cleft (OR 1.19; 95% CI 0.34—4.15). The data from the case-control studies suggested an association between benzodiazepines and major malformations (OR 3.01, 95% CI 1.32—6.84) and oral cleft (OR 1.79; 95% CI 1.13—2.82). The limitations of this meta-analysis included the small number of reports included in the analysis, and that most cases for analyses of both oral cleft and major malformations came from only three studies. A follow up to that meta-analysis included 3 new cohort studies that examined risk for major malformations and one study that considered cardiac malformations. The authors found no new studies with an outcome of oral clefts. After the addition of the new studies, the odds ratio for major malformations with first trimester exposure to benzodiazepines was 1.07 (95% CI 0.91—1.25).
Neonatal Withdrawal and Floppy Infant Syndrome
Neonatal withdrawal syndrome and symptoms suggestive of floppy infant syndrome associated with administration of benzodiazepines during the later stages of pregnancy and peripartum period have been reported. Findings in published scientific literature suggest that the major neonatal side effects of benzodiazepines include sedation and dependence with withdrawal signs. Data from observational studies suggest that fetal exposure to benzodiazepines is associated with the neonatal adverse events of hypotonia, respiratory problems, hypoventilation, low Apgar score, and neonatal withdrawal syndrome.
Animal Data
When midazolam (0, 0.2, 1, or 4 mg/kg/day) was administered intravenously to pregnant rats during the period of organogenesis, no adverse effects on embryofetal development were observed. The highest dose tested, which was associated with minimal evidence of maternal toxicity, is approximately 4 times the maximum recommended human dose (MRHD) of 10 mg based on body surface area (mg/m2).
When midazolam (0, 0.2, 0.6, and 2 mg/kg/day) was administered intravenously to rabbits during the period of organogenesis, no adverse effects on embryofetal development were reported. The high dose, which was not associated with evidence of maternal toxicity, is approximately 4 times the MRHD on a mg/m2 basis.
When midazolam (0, 0.2, 1, or 4 mg/kg/day) was administered intravenously to female rats during late gestation and throughout lactation, no clear adverse effects were noted in the offspring. The high dose, which was not associated with evidence of maternal toxicity, is approximately 4 times the MRHD on a mg/m2 basis.
In published animal studies, administration of benzodiazepines, including midazolam, or other drugs that enhance GABAergic neurotransmission to neonatal rats has been reported to result in widespread apoptotic neurodegeneration in the developing brain at plasma concentrations relevant for seizure control in humans. The window of vulnerability to these changes in rats (postnatal days 0-14) includes a period of brain development corresponding to that taking place during the third trimester of pregnancy in humans.
8.2 Lactation
Risk Summary
Midazolam is excreted in human milk. Studies assessing the effects of midazolam in the breastfed infant or on milk production/excretion have not been performed. Postmarketing experience suggests that breastfed infants of mothers taking benzodiazepines, such as NAYZILAM, may have effects of lethargy, somnolence, and poor sucking.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for NAYZILAM and any potential adverse effects on the breastfed infant from midazolam or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness of NAYZILAM have been evaluated in the age group 12 to 17 years. Use of NAYZILAM in this age group is supported by evidence from an adequate and well-controlled study of NAYZILAM in adults and adolescents with seizure clusters [see Clinical Studies (14)] and pharmacokinetic and safety data from adult and pediatric patients [see Clinical Pharmacology (12.3)].
Safety and effectiveness in pediatric patients below the age of 12 years have not been established.
8.5 Geriatric Use
Safety and efficacy studies of NAYZILAM did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Geriatric patients have longer elimination half-lives for midazolam and its metabolites, which may result in prolonged drug exposure. Geriatric patients may have altered drug distribution; diminished hepatic and/or renal function; and subjects over 70 years of age may be particularly sensitive [see Clinical Pharmacology (12.3)]. Administration of intramuscular (IM) midazolam to elderly patients has been associated with rare reports of death under circumstances compatible with cardiorespiratory depression [see Warnings and Precautions (5.2)]. In most of these cases, the patients also received other CNS depressants capable of depressing respiration, especially narcotics [see Warnings and Precautions (5.1, 5.3)]. Close monitoring of geriatric patients is recommended.
8.6 Renal Impairment
Based on a population pharmacokinetic analysis of patients administered NAYZILAM, midazolam and 1-OH midazolam pharmacokinetics are expected to be similar in subjects with mild renal impairment when compared to normal subjects. Safety and efficacy studies of NAYZILAM did not include patients with severe renal impairment and there were not enough subjects with moderate renal impairment in clinical studies for population pharmacokinetic analysis. Patients with moderate and severe renal impairment may have slower elimination of midazolam and its metabolites, which may result in prolonged drug exposure [see Clinical Pharmacology (12.3)].
8.7 Congestive Heart Failure
Patients with congestive heart failure eliminate midazolam more slowly, which may result in prolonged drug exposure [see Clinical Pharmacology (12.3)].
-
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
NAYZILAM contains the benzodiazepine midazolam, a Schedule IV controlled substance under the Controlled Substances Act.
9.2 Abuse
Benzodiazepines, such as midazolam, may be subject to abuse. Abuse is the intentional non-therapeutic use of a drug, even once, to achieve a desired psychological or physiological effect. Available data concerning the drug abuse and dependence potential of midazolam suggest that its abuse potential is at least equivalent to that of diazepam.
The pharmacological profile of NAYZILAM is similar to that of other benzodiazepines listed in Schedule IV of the Controlled Substance Act, particularly in its potentiation of GABAergic transmission through its action on GABAA receptors, which leads to sedation and somnolence.
Midazolam was actively self-administered in primate models used to assess the positive reinforcing effects of psychoactive drugs. Midazolam produced physical dependence of a mild to moderate intensity in cynomolgus monkeys after 5 to 10 weeks of administration.
Assessment of the abuse-related subjective effects comparing NAYZILAM to oral midazolam syrup was conducted in adult subjects with a history of benzodiazepine recreational drug use. No statistically significant or clinically-relevant differences in subjective positive effects (i.e., Drug Liking, Overall Drug Liking, Take Drug Again, and High) were observed between NAYZILAM and oral midazolam syrup. However, subjective positive effects on all these measures were significantly greater for NAYZILAM than for placebo confirming that NAYZILAM has abuse potential. Somnolence occurred at a similar rate in both midazolam groups, but euphoric mood occurred at a greater rate in NAYZILAM (4 to 16%) compared to the oral midazolam syrup (4 to 8.5%).
9.3 Dependence
Physical dependence is a state of adaptation that is manifested by a specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, decreasing blood levels of the drug, and/or administration of an antagonist.
Benzodiazepines can cause physical dependence. Physical dependence results in withdrawal symptoms in patients who abruptly discontinue the drug. Withdrawal symptoms (i.e., convulsions, hallucinations, tremors, abdominal and muscle cramps, vomiting, and sweating), similar in characteristics to those noted with barbiturates and alcohol, have occurred following abrupt discontinuation of midazolam following chronic administration.
9.4 Chronic Use
NAYZILAM is not recommended for chronic, daily use as an anticonvulsant because of the potential for development of tolerance to midazolam. In clinical trials, patients were treated with NAYZILAM no more frequently than every 3 days.
Chronic daily use of benzodiazepines may increase the frequency and/or severity of tonic-clonic seizures, requiring an increase in the dosage of standard anticonvulsant medication. In such cases, abrupt withdrawal of chronic benzodiazepines may also be associated with a temporary increase in the frequency and/or severity of seizures.
-
10 OVERDOSAGE
Symptoms
The manifestations of midazolam overdosage reported are similar to those observed with other benzodiazepines, including sedation, somnolence, confusion, impaired coordination, diminished reflexes, coma, and untoward effects on vital signs.
Treatment
Treatment of midazolam overdosage is the same as that followed for overdosage with other benzodiazepines. Respiration, pulse rate, and blood pressure should be monitored and general supportive measures should be employed. Attention should be given to the maintenance of a patent airway and support of ventilation, including administration of oxygen. An intravenous infusion should be started. Should hypotension develop, treatment may include intravenous fluid therapy, repositioning, judicious use of vasopressors appropriate to the clinical situation, if indicated, and other appropriate countermeasures. There is no information as to whether peritoneal dialysis, forced diuresis, or hemodialysis are of any value in the treatment of midazolam overdosage.
Flumazenil, a specific benzodiazepine-receptor antagonist, is indicated for the complete or partial reversal of the sedative effects of benzodiazepines and may be used in situations when an overdose with NAYZILAM is known or suspected. There are anecdotal reports of adverse hemodynamic responses associated with midazolam following administration of flumazenil to pediatric patients. Prior to the administration of flumazenil, necessary measures should be instituted to secure the airway, assure adequate ventilation, and establish adequate intravenous access. The reversal of benzodiazepine effects may be associated with the onset of seizures in certain high-risk patients. The prescriber should be aware of a risk of seizure in association with flumazenil treatment, particularly in long-term benzodiazepine users. The administration of flumazenil in cases of benzodiazepine overdose can lead to withdrawal and adverse reactions, including increased seizures. Its use in patients with epilepsy is typically not recommended.
-
11 DESCRIPTION
NAYZILAM contains midazolam, a compound of the benzodiazepine class. Midazolam is chemically designated as 8-Chloro-6-(ο-fluorophenyl)-1-methyl-4H-imidazo[1,5-a][1,4]benzodiazepine, and it has the following structure:
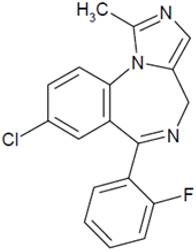
The empirical formula is C18H13ClFN3 representing a molecular weight of 325.8. Midazolam, USP is a white or yellowish, crystalline powder that is practically insoluble in water, soluble in methanol, and freely soluble in acetone and in alcohol.
NAYZILAM nasal spray is a clear, colorless to yellowish colored liquid. Each single-dose NAYZILAM unit is for nasal administration and delivers 5 mg of midazolam in 0.1 mL of solution containing ethanol; PEG-6 methyl ether; polyethylene glycol 400; propylene glycol; and purified water.
The pH range of solution is approximately 5.0 to 9.0.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The exact mechanism of action for midazolam is not fully understood, but it is thought to involve potentiation of GABAergic neurotransmission resulting from binding at the benzodiazepine site of the GABAA receptor.
12.2 Pharmacodynamics
The pharmacodynamic properties of midazolam and its metabolites, are similar to those of other benzodiazepines, including sedative, anxiolytic, amnestic, and hypnotic activities. The effects of midazolam on the CNS are dependent on the dose administered, the route of administration, and the presence or absence of other medications.
Treatment with NAYZILAM was associated with effects on measures of sedation and measures of psychomotor performance [see Warnings and Precautions (5.3)]. Sedation and psychomotor impairment effects generally began to occur within 10 minutes post dose with peak effects observed within 30 minutes to 2 hours post dose. The pharmacodynamic effects generally returned to near baseline levels by 4 hours post-dose.
12.3 Pharmacokinetics
Pharmacokinetics
Based on a population pharmacokinetic analysis, plasma exposures (Cmax and AUC) of midazolam in epilepsy patients increase approximately proportional to dose from 5.0 mg to 15 mg, 0.5 and 1.5 times the recommended maximum total dose (5 mg Initial Dose + 5 mg Second Dose), respectively.
Absorption
Following nasal administration of a single 5 mg midazolam dose to healthy adults, midazolam was absorbed with median Tmax (range) of 17.3 (7.8 to 28.2) minutes; midazolam mean (±SD) Cmax and AUC0-∞ were 54.7 (±30.4) ng/mL and 126.2 (±59) ng∙hr/mL, respectively. The mean absolute bioavailability is approximately 44%.
Distribution
In adults and pediatric patients, midazolam is approximately 97% bound to plasma protein, principally albumin. In healthy volunteers, 1-hydroxy midazolam is bound to the extent of 89%.
The estimated total volume of distribution of midazolam is 226.5 L.
In humans, midazolam has been shown to cross the placenta and enter into fetal circulation and has been detected in human milk and CSF [see Use in Specific Populations (8.1, 8.2)].
Elimination
Following administration of NAYZILAM in clinical trials, median midazolam and 1-hydroxy-midazolam elimination half-lives ranged from 2.1 to 6.2 hours and 2.7 to 7.2 hours, respectively, independent of dose.
Metabolism
Midazolam is primarily metabolized by liver and intestinal cytochrome P450 3A4 (CYP3A4) to its pharmacologic active metabolite, 1-hydroxy midazolam (also termed α-hydroxy-midazolam). Midazolam is also metabolized to two other minor metabolites: 4-hydroxy metabolite and 1,4-dihydroxy metabolite. The principal urinary excretion products are glucuronide conjugates of the hydroxylated derivatives.
Studies of the intravenous administration of 1-hydroxy-midazolam in humans suggest that 1-hydroxy-midazolam is at least as potent as the parent compound and may contribute to the net pharmacologic activity of midazolam.
Specific Populations
Geriatric Patients
In a parallel group study of 2.5 mg and 5 mg doses of NAYZILAM, mean systemic exposure (AUC) and peak plasma concentrations (Cmax) of midazolam were 21 to 45% higher in geriatric subjects (> 65 years old) as compared to non-geriatric subjects. The terminal half-life was increased by approximately 2 hours in the geriatric subjects because of a decrease in clearance [see Use in Specific Populations (8.5)].
Obesity
In a study comparing normal (n=20) and obese patients (n=20), the mean half-life of midazolam administered by parental route was greater in the obese group (5.9 versus 2.3 hours). This was because of an increase of approximately 50% in the volume of distribution (Vd) corrected for total body weight. The clearance was not significantly different between groups.
Patients with Renal Impairment
Patients with renal impairment may have longer elimination half-lives for midazolam and its metabolites [see Use in Specific Populations (8.6)].
Midazolam and 1-hydroxy-midazolam pharmacokinetics in 6 ICU patients who developed acute renal failure (ARF) were compared with a normal renal function control group. Midazolam was administered as an infusion (5 to 15 mg/hr). Midazolam clearance was reduced (1.9 vs 2.8 mL/min/kg) and the half-life was prolonged (7.6 vs 13 hours) in the ARF patients. The renal clearance of the 1-hydroxy-midazolam glucuronide was prolonged in the ARF group (4 vs 136 mL/min) and the half-life was prolonged (12 vs >25 hours). Plasma levels accumulated in all ARF patients to about ten times that of the parent drug. The relationship between accumulating metabolite levels and prolonged sedation is unclear.
In a study of chronic renal failure patients (n=15) receiving a single intravenous dose of midazolam, there was a 2-fold increase in the clearance and volume of distribution, but the half-life remained unchanged.
Patients with Hepatic Impairment
Midazolam pharmacokinetics were studied after an intravenous single dose (0.075 mg/kg) was administered to patients with biopsy proven alcoholic cirrhosis (n=7) and control patients (n=8). The mean half-life of midazolam increased 2.5-fold in the patients with cirrhosis. Clearance was reduced by 50% and the Vd increased by 20%. In another study in male patients with cirrhosis (n=21) without ascites and with normal kidney function as determined by creatinine clearance, no changes in the pharmacokinetics of midazolam or 1-hydroxy-midazolam were observed when compared to healthy individuals. The clinical significance of these findings is unknown.
Drug Interaction Studies
Since NAYZILAM is metabolized by CYP3A4, interactions with drugs that inhibit or induce CYP3A4 are likely.
Inhibitors of CYP3A4 Isozymes
Coadministration of CYP3A4 inhibitors with NAYZILAM has not been studied. However, the effects of inhibitors on midazolam exposure following NAYZILAM administration are expected to be similar to those following IV midazolam administration. Concomitant use of CYP3A4 inhibitors may result in prolonged sedation because of a decrease in plasma clearance of midazolam [Warnings and Precautions (5.3) and Drug Interactions (7.1)].
- The effect of single oral doses of 800 mg cimetidine and 300 mg ranitidine on steady-state concentrations of oral midazolam was examined in a randomized crossover study (n=8). Cimetidine increased the mean midazolam steady-state concentration from 57 to 71 ng/mL. Ranitidine increased the mean steady-state concentration to 62 ng/mL. No change in choice reaction time or sedation index was detected after dosing with the H2 receptor antagonists.
- In a placebo-controlled study, erythromycin administered as a 500 mg dose, three times a day, for 1 week (n=6), reduced the clearance of midazolam following a single 0.5 mg/kg IV dose. The half-life was approximately doubled.
- The effects of diltiazem (60 mg three times a day) and verapamil (80 mg three times a day) on the pharmacokinetics and pharmacodynamics of midazolam were investigated in a three-way crossover study (n=9). The half-life of midazolam increased from 5 to 7 hours when midazolam was taken in conjunction with verapamil or diltiazem. No interaction was observed in healthy subjects between midazolam and nifedipine.
- In a placebo-controlled study, where saquinavir or placebo was administered orally as a 1200 mg dose three times a day for 5 days (n=12), a 56% reduction in the clearance of midazolam following a single 0.05 mg/kg IV dose was observed. The half-life was approximately doubled.
Inducers of CYP3A4 Isozymes
Exposures (e.g., combined Cmax or AUC of midazolam and the 1-OH-midazolam active metabolite) are decreased 16 to 26% when NAYZILAM is co-administered with anti-epileptic drugs that are strong CYP3A4 inducers (e.g., phenytoin, phenobarbital, primidone, carbamazepine). Exposures (e.g., combined Cmax or AUC of midazolam and the 1-OH-midazolam active metabolite) are decreased 8 to 15% when NAYZILAM is co-administered with anti-epileptic drugs that are weak to moderate CYP3A4 inducers (e.g., clobazam, eslicarbazepine, felbamate, oxcarbazepine, rufinamide, topiramate). These changes in exposures are not expected to be clinically significant.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Midazolam maleate was administered in the diet to mice and rats for 2 years at doses of 0, 1, 9, or 80 mg/kg/day. In female mice in the highest dose group there was a marked increase in the incidence of hepatic tumors. In high-dose male rats there was a small but statistically significant increase in benign thyroid follicular cell tumors. The highest dose not associated with increased tumor incidences in mice and rats (9 mg/kg/day) is approximately 4 and 9 times, respectively, the recommended human dose (RHD) of 10 mg based on body surface area (mg/m2). The pathogenesis of induction of these tumors is not known. These tumors were found after chronic administration, whereas human use will ordinarily be of single or several doses.
-
14 CLINICAL STUDIES
The effectiveness of NAYZILAM for the acute treatment of intermittent, stereotypic episodes of frequent seizure activity (i.e., seizure clusters, acute repetitive seizures) that are distinct from a patient's usual seizure pattern in patients with epilepsy 12 years of age and older was established in a randomized, double-blind, placebo-controlled trial (Study 1; NCT 01390220).
Study 1 enrolled patients with epilepsy on a stable regimen of antiepileptic drugs who were identified by their physicians as having intermittent, stereotypic episodes of frequent seizure activity that were distinct from the patient's usual seizure pattern.
Study 1 was conducted in two phases: an open-label Test Dose Phase followed by a randomized, double-blind, placebo-controlled, Comparative Phase. In the Test Dose Phase, tolerability was assessed in 292 patients who, in the absence of a seizure, received two 5 mg doses of NAYZILAM (10 mg total dosage) separated by 10 minutes. Patients were excluded from participation in the Comparative Phase if they failed to meet pre-defined blood pressure, heart rate, sedation, electrocardiogram, and peripheral oxygen saturation criteria.
In the Comparative Phase, 201 patients treated a single seizure cluster episode in an outpatient setting with either a blinded dose of NAYZILAM 5 mg (134 patients) or placebo (67 patients). If the seizure activity persisted or recurred, patients in both groups had the option to receive a subsequent unblinded dose of NAYZILAM 5 mg to be used between 10 minutes and 6 hours after administration of the initial blinded dose of study drug.
The primary efficacy endpoint for Study 1 was treatment success, defined as the termination of seizures within 10 minutes after the initial blinded dose of study drug and the absence of a recurrence of seizures within 6 hours of the initial blinded dose of study drug. A statistically significantly higher percentage of NAYZILAM-treated patients met the primary efficacy endpoint, as shown in Table 4.
Table 4: Primary Endpoint Results: Treatment Success (Study 1) NAYZILAM
(N=134)Placebo
(N=67)Treatment success (%) 53.7 34.3 95% CI (45.3, 62.2) (23.0, 45.7) p-value 0.011 Numerical differences in favor of NAYZILAM were observed on each of the components of the treatment success responder definition; termination of seizure(s) within 10 minutes after initial dose of study drug (80.6 versus 70.1%) and the absence of seizure recurrence between 10 minutes and 6 hours after the initial dose of study drug (58.2 versus 37.3%).
Study 1 also evaluated the occurrence and time to next seizure after the initial blinded dose of study drug. A smaller proportion of NAYZILAM-treated patients experienced the next seizure within 24 hours after the initial blinded dose of study drug (37.3% versus 46.3%). NAYZILAM-treated patients experienced a statistically longer time-to-next-seizure than the placebo group (Figure 1).
FIGURE 1: Kaplan-Meier Analysis of Time-to-Next-Seizure (Study 1)
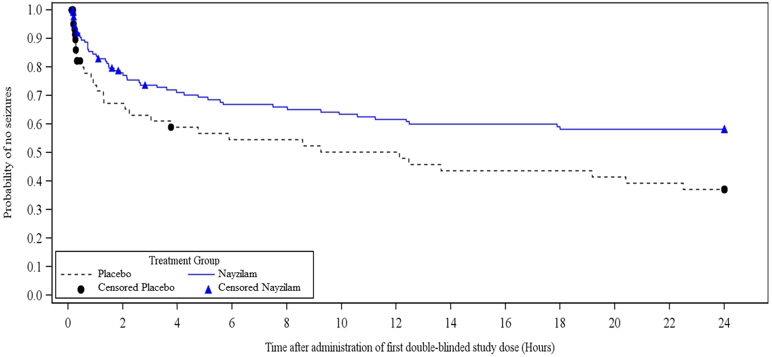
Analysis by gender revealed no substantial differences in treatment response. Informative subgroup analyses by age and race were not possible because of the small percentage of patients less than 18 years of age or 65 years of age or greater, and of non-White patients in the study.
-
16 HOW SUPPLIED / STORAGE AND HANDLING
16.1 How Supplied
NAYZILAM is supplied as a solution of midazolam. Each single-dose nasal spray unit delivers 5 mg of midazolam in 0.1 mL of solution.
NAYZILAM is supplied in boxes of 2 nasal spray units (NDC: 50474-500-15), each contained within an individual blister pack.
-
17 PATIENT COUNSELING INFORMATION
Advise patients and caregivers to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Risks from Concomitant Use with Opioids
Inform patients and caregivers that potentially fatal additive effects may occur if NAYZILAM is used with opioids and not to use NAYZILAM concomitantly with opioids unless supervised by a healthcare provider. If a decision is made to prescribe NAYZILAM concomitantly with opioids, instruct caregivers to follow patients closely for signs and symptoms of respiratory depression and sedation [see Warnings and Precautions (5.1)].
Risks of Cardiorespiratory Adverse Reactions
Warn patients and caregivers about the risks of respiratory depression, cardiac and respiratory arrest [see Warnings and Precautions (5.2)].
Advise caregivers on the signs and symptoms of respiratory depression to look for, how long to observe patients after administering NAYZILAM, the circumstances under which a second dose should not be given, and the circumstances under which emergency medical care should be summoned [see Dosage and Administration (2.1)].
CNS Depression from Concomitant Use with Other CNS Depressants
Warn patients and caregivers that the use of NAYZILAM in combination with alcohol or other CNS depressant drugs may increase the risk of hypoventilation, airway obstruction, desaturation, or apnea and may contribute to profound and/or prolonged drug effect [see Warnings and Precautions (5.3)].
Caution patients against engaging in hazardous occupations requiring mental alertness, such as operating machinery, driving a motor vehicle or riding a bicycle until they have completely returned to their level of baseline functioning.
Suicidal Behavior and Ideation
Counsel patients, their caregivers, and families that AEDs, including NAYZILAM, may increase the risk of suicidal thoughts and behavior and that they should be alert for the emergence or worsening of the signs and symptoms of depression, any unusual changes in mood or behavior or the emergence of suicidal thoughts, behavior or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers [see Warnings and Precautions (5.4)].
Impaired Cognitive Function
Warn patients that midazolam, including NAYZILAM, is associated with a high incidence of partial or complete impairment of recall for the next several hours. Counsel patients on when they can engage in activities requiring complete mental alertness, operate hazardous machinery, or drive a motor vehicle after taking NAYZILAM [see Warnings and Precautions (5.5)].
Pregnancy
Instruct patients to inform their physician if they are pregnant or are planning to become pregnant. Several studies have suggested an increased risk of congenital malformations associated with the use of benzodiazepine drugs. Animal studies have demonstrated an effect on early brain development and long-term cognitive effects with exposure to anesthetic and sedation drugs in the third trimester of gestation. Encourage patients to enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry if they become pregnant. The registry is collecting information about the safety of antiepileptic drugs during pregnancy [see Use in Specific Populations (8.1)].
Lactation
Instruct patients to inform their physicians if they are nursing [see Use in Specific Populations (8.2)].
Important Treatment Instructions
Instruct patients and caregivers on what is and is not an intermittent and stereotypic episode of increased seizure activity (i.e., seizure cluster) that is appropriate for treatment, and the timing of administration in relation to the onset of the episode.
Instruct patients and caregivers on what to observe following administration, and what would constitute an outcome requiring immediate medical attention.
Instruct patients and caregivers not to administer a second dose of NAYZILAM if they are concerned by the patient's breathing, the patient requires emergency rescue treatment with assisted breathing or intubation, or there is excessive sedation [see Dosage and Administration (2.1)].
Advise patients and caregivers on how frequently they can treat successive seizure cluster episodes over time.
- SPL UNCLASSIFIED SECTION
-
MEDICATION GUIDE
This Medication Guide has been approved by the U.S. Food and Drug Administration Issued: 5/2019 MEDICATION GUIDE
NAYZILAM® (NAY-zil-am)
(midazolam) nasal spray, CIVWhat is the most important information I should know about NAYZILAM?
NAYZILAM is a benzodiazepine medicine. Taking benzodiazepines with opioid medicines, alcohol, or other central nervous system depressants (including street drugs) can cause severe drowsiness, breathing problems (respiratory depression), coma, and death.
NAYZILAM may cause serious breathing problems and excessive sleepiness (sedation). Get emergency medical help right away if any of the following occur:- unusually shallow or slowed breathing
- stop breathing, which may lead to your heart stopping
- unusually excessive sleepiness
- thoughts about suicide or dying
- feeling agitated or restless
- acting aggressive, being angry, or violent
- attempts to commit suicide
- panic attacks
- acting on dangerous impulses
- new or worse depression
- trouble sleeping (insomnia)
- an extreme increase in activity and talking (mania)
- new or worse anxiety
- new or worse irritability
- other unusual changes in behavior or mood
How can I watch for early symptoms of suicidal thoughts or actions? - Pay attention to any changes, especially sudden changes in mood, behaviors, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled.
Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes.What is NAYZILAM? - NAYZILAM is a prescription medicine used for the short-term treatment of seizure clusters (also known as "acute repetitive seizures") in patients 12 years of age and older.
- NAYZILAM is a federal controlled substance (C-IV) because it can be abused or lead to dependence. Keep NAYZILAM in a safe place to prevent misuse and abuse. Selling or giving away NAYZILAM may harm others and is against the law. Tell your healthcare provider if you have abused or been dependent on alcohol, prescription drugs, or street drugs.
- It is not known if NAYZILAM is safe and effective in children under 12 years of age.
Do not use NAYZILAM if you: - are allergic to midazolam.
- have an eye problem called acute narrow-angle glaucoma.
Before you use NAYZILAM, tell your healthcare provider about all your medical conditions, including if you: - have a history of depression, mood problems or suicidal thoughts or behavior.
- have asthma, emphysema, bronchitis, chronic obstructive pulmonary disease, or other breathing problems.
- have kidney or liver problems.
- have congestive heart failure.
- have a history of drug or alcohol abuse.
- are pregnant or plan to become pregnant. NAYZILAM may harm your unborn baby.
- Babies born to mothers receiving benzodiazepine medicines (including NAYZILAM) late in pregnancy may be at risk of having breathing problems, feeding problems, dangerously low body temperature, and withdrawal symptoms.
- If you become pregnant while using NAYZILAM, talk to your healthcare provider about registering with the North American Antiepileptic Drug (NAAED) Pregnancy Registry. You can register by calling 1-888-233-2334. For more information about the registry, go to http://www.aedpregnancyregistry.org. The purpose of this registry is to collect information about the safety of antiepileptic drugs during pregnancy.
- are breastfeeding or plan to breastfeed. Midazolam passes into your breast milk and may harm your baby. Talk to your healthcare provider about the best way to feed your baby if you use NAYZILAM.
How should I use NAYZILAM? - Use NAYZILAM in the nose only.
- Use NAYZILAM exactly as your healthcare provider tells you to use it and follow the Instructions for Use that comes with this Medication Guide.
- Your healthcare provider has prescribed NAYZILAM to treat a type of seizure called a "seizure cluster".
- If the seizure cluster is continuing 10 minutes after the first dose of NAYZILAM, a second dose of NAYZILAM may be used if you have been told to do so by your healthcare provider.
- If a second dose of NAYZILAM is used, give the second dose in the other nostril.
- Do not give more than 2 doses of NAYZILAM to treat a seizure cluster.
- If the seizures do not stop after NAYZILAM is used, get emergency medical help right away.
- Do not use NAYZILAM for more than one seizure cluster episode every 3 days. Do not use NAYZILAM for more than five seizure cluster episodes per month.
- If benzodiazepines are stopped after a person takes them daily, they can cause withdrawal symptoms. Stopping benzodiazepines suddenly can cause seizures that will not stop (status epilepticus), hearing or seeing things that are not there (hallucinations), shaking, nervousness, and stomach and muscle cramps. NAYZILAM is not intended to be taken daily.
- If you use too much NAYZILAM, call your healthcare provider or go to the nearest emergency room right away.
What should I avoid while using NAYZILAM? - Do not drive, operate machinery, or do other activities that require mental alertness until you know how NAYZILAM affects you.
- Do not drink alcohol or take opioid medicines or other medicines that make you sleepy or dizzy while taking NAYZILAM until you talk to your healthcare provider. When taken with alcohol or medicines that can cause sleepiness or dizziness, NAYZILAM may make your sleepiness or dizziness worse.
What are the possible side effects of NAYZILAM?
NAYZILAM may cause serious side effects, including:- See "What is the most important information I should know about NAYZILAM?" for information on serious side effects of:
- interaction with other central nervous system depressants
- serious breathing problems and excessive sleepiness
- suicidal thoughts or actions
- Impaired mental alertness including memory problems. See "What should I avoid while using NAYZILAM?"
- Increase in eye pressure in people with acute narrow-angle glaucoma. See "Do not take NAYZILAM if you:"
- sleepiness
- headache
- runny nose
- nasal discomfort
- throat irritation
These are not all the possible side effects of NAYZILAM. Call your doctor for medical advice about side effects.
You may report side effects to FDA at 1-800-FDA-1088.How should I store NAYZILAM? - Store NAYZILAM at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep NAYZILAM in the blister package until ready to use.
General information about the safe and effective use of NAYZILAM.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use NAYZILAM for a condition for which it was not prescribed. Do not give NAYZILAM to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about NAYZILAM that is written for health professionals.What are the ingredients in NAYZILAM?
Active ingredient: midazolam
Inactive ingredients: ethanol, PEG-6 methyl ether, polyethylene glycol 400, propylene glycol and purified water
Manufactured for UCB, Inc., Smyrna, GA 30080.
NAYZILAM® is a registered trademark of UCB Biopharma SPRL.
©2019. All rights reserved.
For more information, go to www.nayzilam.com or call 1-844-599-2273. -
INSTRUCTIONS FOR USE
Instructions for Use
NAYZILAM® (NAY-zil-am)
(midazolam) nasal spray, CIVYou and your family members or caregivers should read this Instructions for Use before you start using NAYZILAM nasal spray and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment. If you and your family members or caregivers have any questions about NAYZILAM, ask your healthcare provider or pharmacist.
Important: NAYZILAM is for use in the nose only. - There is only 1 dose of NAYZILAM in the nasal spray unit.
- Do not try to test or prime the nasal spray unit before use. You will lose the dose.
- Do not open the blister packaging until ready to use.
- Do not use if the nasal spray unit appears damaged.
- Do not use past the expiration date printed on the blister packaging.
- Throw away (dispose of) the nasal spray unit after use.
How to use NAYZILAM nasal spray:
Step 1: Peel open the blister packaging- When ready to use, open the blister packaging.
- Hold blister packaging in the palm of your hand.
- On the foil backing, find the "Peel Here" tab and pull down (see Figure 1).
- Remove the nasal spray unit carefully.
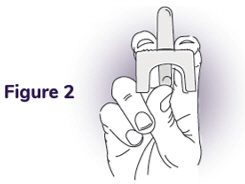
Step 2: Hold the nasal spray unit - Hold the nasal spray unit with your thumb on the plunger and your middle and index fingers on each side of the nozzle (see Figure 2).
- Do not press the plunger yet. If you press the plunger now, you will lose the dose.
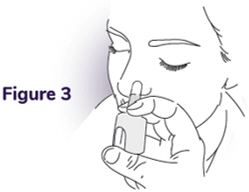
Step 3: Place the tip into 1 nostril - Place the tip of the nozzle into 1 nostril until your fingers on either side of the nozzle touches the bottom of the nose (see Figure 3).
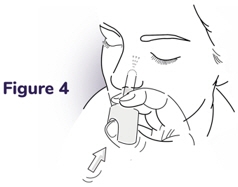
Step 4: Press the plunger - Press the plunger firmly to deliver the dose of NAYZILAM nasal spray (see Figure 4).
- Make sure to firmly press the plunger using 1 motion.
What to do after the NAYZILAM nasal spray has been used:
Remove the nozzle from the nostril after giving the dose.
Note: The plunger will remain inside the nasal spray unit after the dose is given.
Throw away (dispose of) the nasal spray unit and blister packaging in the trash.
What to do if a Second Dose is needed:
Important: If the seizure cluster is continuing 10 minutes after the first dose of NAYZILAM, a second dose of NAYZILAM may be used if you have been told to do so by your healthcare provider.
If you need to give a second dose of NAYZILAM, follow the instructions in this Instructions for Use using a new nasal spray unit in the other nostril. (Repeat Step 1 through Step 4)

Call for help if any of the following happens: - Seizure or seizures continue after giving NAYZILAM to the person as instructed by the healthcare provider.
Local Emergency Number: - Seizure behavior in the person is different from other episodes.
Healthcare Provider's Number: - You are alarmed by the number or severity of the seizure or seizures in the person.
Information for Emergency Responder - You are alarmed by the color or breathing of the person.
Time of first NAYZILAM
dose:________________
Time of second NAYZILAM dose (if
given):______Manufactured for UCB, Inc., Smyrna, GA 30080.
NAYZILAM® is a registered trademark of UCB Biopharma SPRL. ©2019. All rights reserved.
For more information, go to www.nayzilam.com or call 1-844-599-2273.
This Instructions for Use has been approved by the U.S. Food and Drug Administration
Issued: 5/2019 -
PRINCIPAL DISPLAY PANEL - 5 mg Vial Blister Pack Carton
NDC: 50474-500-15
Rx onlyNayzilam® CIV
(midazolam) nasal spray
5 mgFOR NASAL USE ONLY
2 Nasal Spray Units
(1 dose per unit)DO NOT test or prime before use.
-
INGREDIENTS AND APPEARANCE
NAYZILAM
midazolam sprayProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 50474-500 Route of Administration NASAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength midazolam (UNII: R60L0SM5BC) (midazolam - UNII:R60L0SM5BC) midazolam 5 mg in 0.1 mL Inactive Ingredients Ingredient Name Strength PEG-6 methyl ether (UNII: WXH089JZ5E) polyethylene glycol 400 (UNII: B697894SGQ) propylene glycol (UNII: 6DC9Q167V3) water (UNII: 059QF0KO0R) alcohol (UNII: 3K9958V90M) Product Characteristics Color YELLOW (colorless to yellowish) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50474-500-15 2 in 1 CARTON 05/17/2019 1 NDC: 50474-500-14 1 in 1 BLISTER PACK 1 0.1 mL in 1 VIAL, SINGLE-DOSE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA211321 05/17/2019 Labeler - UCB, Inc. (028526403) Registrant - UCB, Inc. (043546522)
Trademark Results [Nayzilam]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 NAYZILAM 88312000 not registered Live/Pending |
UCB Biopharma SPRL 2019-02-22 |
 NAYZILAM 86419161 5386953 Live/Registered |
UCB BIOPHARMA SPRL 2014-10-09 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.