GONITRO- nitroglycerin powder
GONITRO by
Drug Labeling and Warnings
GONITRO by is a Prescription medication manufactured, distributed, or labeled by Allegis Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use GONITRO safely and effectively. See full prescribing information for GONITRO.
GONITRO (nitroglycerin) sublingual powder
Initial U.S. Approval:1981
INDICATIONS AND USAGE
GONITRO is a nitrate vasodilator indicated for acute relief of an attack or prophylaxis of angina pectoris due to coronary artery disease. (1)
DOSAGE AND ADMINISTRATION
- At the onset of an attack, administer one or two packets (400 mcg each) under the tongue. One additional packet may be administered every 5 minutes as needed. No more than three total packets (1200 mcg) are recommended within a 15 minute period. (2.1)
- If chest pain persists after three packets, advise prompt medical attention. (2.1)
- May be used prophylactically 5 to 10 minutes prior to engaging in activities that might precipitate an acute attack. (2.1)
DOSAGE FORMS AND STRENGTHS
Sublingual powder, 400 mcg per packet. (3)
CONTRAINDICATIONS
- Use of phosphodiesterase type 5 (PDE-5) inhibitors, such as avanafil, sildenafil, tadalafil, or vardenafil, or soluble guanylate cyclase (sGC) stimulators. (4.1, 7.1)
- Severe anemia. (4.2)
- Increased intracranial pressure. (4.3)
- Hypersensitivity to GONITRO or to other nitrates or nitrites or any excipient. (4.4)
- Circulatory failure and shock. (4.5)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
Most common adverse reactions occurring at a frequency greater than 2% are headache, dizzinesss and paresthesia. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Allegis Pharmaceuticals, LLC at 1-866-633-9033 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 7/2019
- At the onset of an attack, administer one or two packets (400 mcg each) under the tongue. One additional packet may be administered every 5 minutes as needed. No more than three total packets (1200 mcg) are recommended within a 15 minute period. (2.1)
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 PDE-5-Inhibitors and sGC-Stimulators
4.2 Severe Anemia
4.3 Increased Intracranial Pressure
4.4 Hypersensitivity
4.5 Circulatory Failure and Shock
5 WARNINGS AND PRECAUTIONS
5.1 Tolerance
5.2 Hypotension
5.3 Hypertrophic Obstructive Cardiomyopathy
5.4 Headache
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 PDE-5-Inhibitors and sGC-Stimulators
7.2 Antihypertensives
7.3 Ergotamine
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
10.1 Signs and Symptoms, Methemoglobinemia
10.2 Treatment of Overdosage
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis,Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
Administer one or two packets (400 mcg of nitroglycerin per packet) at the onset of an attack under the tongue. One additional packet may be administered every five minutes as needed. No more than three packets are recommended within a 15-minute period. If the chest pain persists after a total of threepackets, seek prompt medical attention. GONITRO may be used prophylactically 5 to 10 minutes prior to engaging in activities that might precipitate an acute attack.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
4.1 PDE-5-Inhibitors and sGC-Stimulators
Do not use GONITRO in patients who are taking PDE-5-Inhibitors, such as avanafil, sildenafil, tadalafil, or vardenafil. Concomitant use can cause severe hypotension, syncope, or myocardial ischemia [see Drug Interactions (7.1)].
Do not use GONITRO in patients who are taking soluble guanylate cyclase (sGC) stimulators, such as riociguat. Concomitant use can cause hypotension.
4.2 Severe Anemia
GONITRO is contraindicated in patients with severe anemia (large doses of nitroglycerin may cause oxidation of hemoglobin to methemoglobin and could exacerbate anemia).
4.3 Increased Intracranial Pressure
GONITRO may precipitate or aggravate increased intracranial pressure and thus should not be used in patients with possible increased intracranial pressure (e. g. cerebral hemorrhage or traumatic brain injury).
-
5 WARNINGS AND PRECAUTIONS
5.1 Tolerance
Excessive use may lead to the development of tolerance. Only the smallest number of doses required for effective relief of the acute angina attack should be used [see Dosage and Administration (2.1)].
5.2 Hypotension
Severe hypotension, particularly with upright posture, may occur even with small doses of nitroglycerin particularly in patients with constrictive pericarditis, aortic or mitral stenosis, patients who may be volume-depleted, or are already hypotensive. Hypotension induced by nitroglycerin may be accompanied by paradoxical bradycardia and increased angina pectoris. Symptoms of severe hypotension (nausea, vomiting, weakness, pallor, perspiration and collapse/syncope) may occur even with therapeutic doses.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Adverse reactions occurring at a frequency greater than 2% and greater than placebo included: headache, dizziness, and paresthesia.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of GONITRO and other nitroglycerin drugs. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency reliably or establish a causal relationship to drug exposure.
Neurologic: weakness, drowsiness
Dermatologic: cutaneous vasodilation, flushing, drug rash, exfoliative dermatitits
Gastrointestinal: nausea, vomiting
Respiratory: transient hypoxemia
Cardiovascular: tachycardia -
7 DRUG INTERACTIONS
7.1 PDE-5-Inhibitors and sGC-Stimulators
GONITRO is contraindicated in patients who are using a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE-5). PDE-5-Inhibitors such as avanafil, sildenafil, vardenafil, and tadalafil have been shown to potentiate the hypotensive effects of organic nitrates.
GONITRO is contraindicated in patients who are taking soluble guanylate cyclase (sGC) stimulators. Concomitant use can cause hypotension.
The time course and dose dependence of these interactions have not been studied, and use within a few days of one another is not recommended. Appropriate supportive care for the severe hypotension has not been studied, but it seems reasonable to treat this as a nitrate overdose, with elevation of the extremities and with central volume expansion.
7.2 Antihypertensives
Patients receiving antihypertensive drugs, beta-adrenergic blockers, and nitrates should be observed for possible additive hypotensive effects. Marked orthostatic hypotension has been reported when calcium channel blockers and organic nitrates were used concomitantly.
Beta-adrenergic blockers blunt the reflex tachycardia produced by nitroglycerin without preventing its hypotensive effects. If beta-blockers are used with nitroglycerin in patients with angina pectoris, additional hypotensive effects may occur.
7.3 Ergotamine
Oral administration of nitroglycerin markedly decreases the first-pass metabolism of dihydroergotamine and subsequently increases its oral bioavailability. Ergotamine is known to precipitate angina pectoris. Therefore, patients receiving sublingual nitroglycerin should avoid ergotamine and related drugs or be monitored for symptoms of ergotism if this is not possible.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk summary
Limited published data on the use of nitroglycerin are insufficient to determine a drug associated risk of major birth defects or miscarriage. In animal reproduction studies, there were no adverse developmental effects when nitroglycerin was administered intravenously to rabbits or intraperitoneally to rats during organogenesis at doses greater than 64-times the human dose [see Data].
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
No embryotoxic or postnatal development effects were observed with transdermal application in pregnant rabbits and rats at doses up to 240 mg/kg/day for 13 days, at intraperitoneal doses in pregnant rats up to 20 mg/kg/day for 11 days, and at intravenous doses in pregnant rabbits up to 4 mg/kg/day for 13 days.
8.2 Lactation
Risk summary
Sublingual nitroglycerin has not been studied in lactating women. It is not known if nitroglycerin is present in human milk or if nitroglycerin has effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for nitroglycerin and any potential adverse effects on the breastfed child from nitroglycerin or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness of nitroglycerin in pediatric patients have not been established.
8.5 Geriatric Use
Clinical studies did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between elderly (greater than or equal to 65 years) and younger (less than 65 years) patients. In general, dose selection for an elderly patient should start at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
10 OVERDOSAGE
10.1 Signs and Symptoms, Methemoglobinemia
Nitrate overdosage may result in: severe hypotension, persistent throbbing headache, vertigo, palpitation, visual disturbance, flushing and perspiring skin (later becoming cold and cyanotic), nausea and vomiting (possibly with colic and even bloody diarrhea), syncope (especially in the upright posture), methemoglobinemia with cyanosis and anorexia, initial hyperpnea, dyspnea and slow breathing, slow pulse (dicrotic and intermittent), heart block, increased intracranial pressure with cerebral symptoms of confusion and moderate fever, paralysis and coma followed by clonic convulsions, and possibly death due to circulatory collapse.
Case reports of clinically significant methemoglobinemia are rare at conventional doses of organic nitrates. The formation of methemoglobin is dose-related and in the case of genetic abnormalities of hemoglobin that favor methemoglobin formation, even conventional doses of organic nitrates could produce harmful concentrations of methemoglobin.
10.2 Treatment of Overdosage
As hypotension associated with nitroglycerin overdose is the result of venodilatation and arterial hypovolemia, prudent therapy in this situation should be directed toward increase in central fluid volume. No specific antagonist to the vasodilator effects of nitroglycerin is known. Keep the patient recumbent in a shock position and comfortably warm. Passive movement of the extremities may aid venous return. Intravenous infusion of normal saline or similar fluid may also be necessary. Administer oxygen and artificial ventilation, if necessary. If methemoglobinemia is present, administration of methylene blue (1% solution), 1-2 mg per kilogram of body weight intravenously, may be required unless the patient is known to have G-6-PD deficiency. If an excessive quantity of GONITRO has been recently swallowed gastric lavage may be of use.
As epinephrine is ineffective in reversing the severe hypotensive events associated with overdosage, it is not recommended for resuscitation.
-
11 DESCRIPTION
Nitroglycerin, an organic nitrate, is a vasodilator which has effects on both arteries and veins. The chemical name for nitroglycerin is 1,2,3-propanetriol trinitrate (C3H5N3O9). The compound has a molecular weight of 227.09. The chemical structure is:
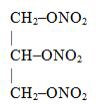
GONITRO is a powder containing nitroglycerin. Each packet of GONITRO delivers 400 mcg of nitroglycerin when administered under the tongue. The sublingual powder is formulated with the following inactive ingredients: anhydrous dibasic calcium phosphate, isomalt, medium-chain triglycerides, peppermint oil, and oleoyl polyoxylglycerides.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Nitroglycerin forms free radical nitric oxide (NO), which activates guanylate cyclase, resulting in an increase of guanosine 3',5'-monophosphate (cyclic GMP) in smooth muscle and other tissues. This eventually leads to dephosphorylation of myosin light chains, which regulates the contractile state in smooth muscle and results in vasodilatation.
12.2 Pharmacodynamics
The principal pharmacological action of nitroglycerin is relaxation of vascular smooth muscle. Although venous effects predominate, nitroglycerin produces, in a dose-related manner, dilation of both arterial and venous beds. Dilation of the postcapillary vessels, including large veins, promotes peripheral pooling of blood, decreases venous return to the heart, and reduces left ventricular end-diastolic pressure (preload). Nitroglycerin also produces arteriolar relaxation, thereby reducing peripheral vascular resistance and arterial pressure (after load), and dilates large epicardial coronary arteries; however, the extent to which this latter effect contributes to the relief of exertional angina is unclear.
Therapeutic doses of nitroglycerin may reduce systolic, diastolic and mean arterial blood pressure. Effective coronary perfusion pressure is usually maintained, but can be compromised if blood pressure falls excessively or increased heart rate decreases diastolic filling time.
Elevated central venous and pulmonary capillary wedge pressures, and pulmonary and systemic vascular resistance are also reduced by nitroglycerin therapy. Heart rate is usually slightly increased, presumably a reflex response to the fall in blood pressure. Cardiac index may be increased, decreased, or unchanged. Myocardial oxygen consumption or demand (as measured by the pressure-rate product, tension-time index, and stroke-work index) is decreased and a more favorable supply-demand ratio can be achieved. Patients with elevated left ventricular filling pressure and increased systemic vascular resistance in association with a depressed cardiac index are likely to experience an improvement in cardiac index. In contrast, when filling pressures and cardiac index are normal, cardiac index may be slightly reduced following nitroglycerin administration.
12.3 Pharmacokinetics
In a pharmacokinetic study a single 800 mcg dose of GONITRO was administered sublingually to healthy volunteers (n = 32), the mean Cmax and tmax for GTN were 1,700 pg/mL and 7 minutes, respectively. Additionally, in these subjects the mean area under the curve (AUC) for GTN was 12,300 pg/mL . min.
The results of the study indicate that the sublingual absorption of GTN is higher following the administration of GONITRO compared to Nitrolingual® Pumpspray.
A liver reductase enzyme is of primary importance in the metabolism of nitroglycerin to glycerol di- and mononitrate metabolites and ultimately to glycerol and organic nitrate. Known sites of extrahepatic metabolism include red blood cells and vascular walls. In addition to nitroglycerin, 2 major metabolites, 1,2- and 1,3-dinitroglycerin are found in plasma. The mean elimination half-life of both 1,2- and 1,3-dinitroglycerin is about 40 minutes. The 1,2- and 1,3-dinitroglycerin metabolites have been reported to possess some pharmacological activity, whereas the glycerol mononitrate metabolites of nitroglycerin are essentially inactive. Higher plasma concentrations of the dinitro metabolites, with their nearly 8-fold longer elimination half-lives, may contribute significantly to the duration of pharmacologic effect.
The volume of distribution of nitroglycerin following intravenous administration is 3.3 L/kg.
Drug interactions
Aspirin: Coadministration of nitroglycerin with high dose aspirin (1000 mg) results in increased exposure to nitroglycerin. The vasodilatory and hemodynamic effects of nitroglycerin may be enhanced by concomitant administration of nitroglycerin with high dose aspirin.
Tissue-type plasminogen activator (t-PA): Concomitant administration of t-PA and intravenous nitroglycerin has been shown to reduce plasma levels of t-PA and its thrombolytic effect.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis,Mutagenesis, Impairment of Fertility
Animal carcinogenesis studies with sublingual nitroglycerin have not been performed.
Rats receiving up to 434 mg/kg/day of dietary nitroglycerin for 2 years developed dose-related fibrotic and neoplastic changes in liver, including carcinomas, and interstitial cell tumors in testes. At high dose, the incidences of hepatocellular carcinomas in both sexes were 52 % vs. 0 % in controls, and incidences of testicular tumors were 52 % vs. 8 % in controls. Lifetime dietary administration of up to 1058 mg/kg/day of nitroglycerin was not tumorigenic in mice.
Nitroglycerin was weakly mutagenic in Ames tests performed in two different laboratories. There was no evidence of mutagenicity in an in vivo dominant lethal assay with male rats treated with doses up to about 363 mg/kg/day, p.o., or in in vitro cytogenic tests in rat and dog tissues and for chromosomal aberration in Chinese hamster ovary cells.
In a three-generation reproduction study, rats received dietary nitroglycerin at doses up to about 434 mg/kg/day for six months prior to mating of the F0 generation with treatment continuing through successive F1 and F2 generations. The high dose was associated with decreased feed intake and body weight gain in both sexes at all matings. No specific effect on the fertility of the F0 generation was seen. Infertility noted in subsequent generations, however, was attributed to increased interstitial cell tissue and aspermatogenesis in the high-dose males. In this three-generation study there was no clear evidence of teratogenicity.
-
14 CLINICAL STUDIES
In a randomized, double-blind single-dose, 5-period cross-over study in 51 patients with exertional angina pectoris significant dose-related increases in exercise tolerance, time to onset of angina and ST-segment depression were seen following doses of 200-1600 mcg of sublingual nitroglycerin as compared to placebo.
The drug showed a profile of mild to moderate adverse events.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Each box of GONITRO (nitroglycerin) sublingual powder, contains 12, 36 or 96 packets.
Each packet contains 400 mcg of nitroglycerin.
GONITRO is available as:
Box of 12 packets NDC: 28595-705-12
Box of 36 packets NDC: 28595-705-36
Box of 96 packets NDC: 28595-705-96Store at 20°C - 25°C (68°F - 77°F); excursions permitted between 5°C – 40°C (41°F – 104°F).
Rx Only.
- 17 PATIENT COUNSELING INFORMATION
-
PATIENT PACKAGE INSERT
Instructions for Use
GONITRO (GO-NYE-troh)
(nitroglycerin)
sublingual powderRead these Instructions for Use before you start taking GONITRO and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
Important information:
- GONITRO is for use under the tongue.
- A dose of GONITRO may be either 1 or 2 packets. Follow your healthcare provider’s instructions about how many packets you should take for each dose. After you take your first dose of GONITRO, you may take 1 additional packet of GONITRO every 5 minutes, if needed.
- You should not take more than 3 packets of GONITRO within 15 minutes.
- Get emergency medical help right away if you still have chest pain after taking a total of 3 packets of GONITRO.
- It is best to take GONITRO while you are resting and in a sitting position.
Taking a dose of GONITRO
Step 1. Hold the packet of GONITRO upright with the notch and red arrow line at the top of the packet.
Step 2. Tap the bottom of the GONITRO packet so that the powder settles at the bottom of the packet.
Step 3. Hold the GONITRO packet at the notch. Hold the packet as close to your mouth as possible and tear along the red arrow line.
Step 4. Lift up your tongue.
Step 5. Pour all of the powder in the GONITRO packet under your tongue.
Step 6. Close your mouth right away and breathe normally through your nose. Allow all of the powder to dissolve before you swallow. Do not rinse your mouth or spit for 5 minutes after taking GONITRO.
Step 7. If another dose of GONITRO is needed, repeat Steps 1 through 6.- Regularly check the number of packets remaining in your GONITRO box and refill your prescription when there are three packets remaining.
How should I store GONITRO
Store GONITRO at room temperature between 68°F to 77°F (20°C to 25°C).
Keep GONITRO and all medicines out of the reach of children.
These Instructions for Use have been approved by the U.S. Food and Drug Administration.
Manufactured for
Allegis Pharmaceuticals, LLC
Canton, MS 39046
by G. Pohl-Boskamp GmbH & Co. KG
25551 Hohenlockstedt, Germany.Issued: 07/2019
-
PRINCIPAL DISPLAY PANEL
Prinicpal Display PanelNDC: 28595-705-12
GONITRO®
(nitroglycerin)
sublingual powder
400 mcg per packet
12 single dose packets
Prinicpal Display Panel
NDC: 28595-705-36
GONITRO®
(nitroglycerin)
sublingual powder
400 mcg per packet
36 single dose packets
Prinicpal Display Panel
NDC: 28595-705-96
GONITRO®
(nitroglycerin)
sublingual powder
400 mcg per packet
96 single dose packets
-
INGREDIENTS AND APPEARANCE
GONITRO
nitroglycerin powderProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 28595-705 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NITROGLYCERIN (UNII: G59M7S0WS3) (NITROGLYCERIN - UNII:G59M7S0WS3) NITROGLYCERIN 400 ug Inactive Ingredients Ingredient Name Strength ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) ISOMALT (UNII: S870P55O2W) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PEPPERMINT OIL (UNII: AV092KU4JH) PEG-5 oleate (UNII: 0240V77G50) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 28595-705-36 36 in 1 BOX 07/01/2019 1 1 in 1 PACKET; Type 0: Not a Combination Product 2 NDC: 28595-705-99 3 in 1 BOX 07/01/2019 2 1 in 1 PACKET; Type 0: Not a Combination Product 3 NDC: 28595-705-96 96 in 1 BOX 07/01/2019 3 1 in 1 PACKET; Type 0: Not a Combination Product 4 NDC: 28595-705-12 12 in 1 BOX 07/01/2019 4 NDC: 28595-705-01 1 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA208424 07/01/2019 Labeler - Allegis Pharmaceuticals, Inc. (792272861)
Trademark Results [GONITRO]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 GONITRO 98060845 not registered Live/Pending |
Desmoid A.G. 2023-06-27 |
 GONITRO 86725748 5110730 Live/Registered |
G. Pohl-Boskamp GmbH & Co. KG 2015-08-14 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.