GLIMEPIRIDE by DIRECT RX GLIMEPIRIDE tablet
GLIMEPIRIDE by
Drug Labeling and Warnings
GLIMEPIRIDE by is a Prescription medication manufactured, distributed, or labeled by DIRECT RX. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- 1 INDICATIONS AND USAGE
- 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
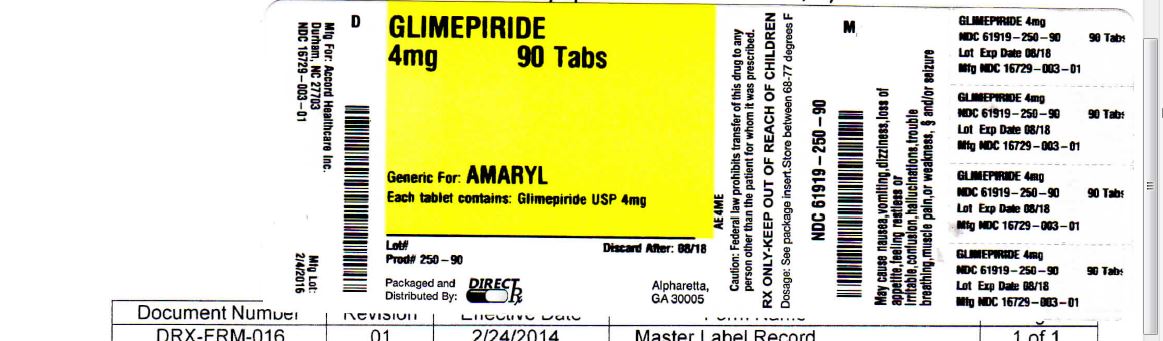
- PACKAGE LABEL PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GLIMEPIRIDE
glimepiride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 61919-250(NDC:16729-003) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLIMEPIRIDE (UNII: 6KY687524K) (GLIMEPIRIDE - UNII:6KY687524K) GLIMEPIRIDE 4 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) POVIDONE (UNII: FZ989GH94E) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) Product Characteristics Color blue Score 2 pieces Shape OVAL Size 11mm Flavor Imprint Code AHI;4 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 61919-250-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 02/04/2016 2 NDC: 61919-250-04 4 in 1 BOTTLE; Type 0: Not a Combination Product 02/04/2016 3 NDC: 61919-250-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 02/04/2016 4 NDC: 61919-250-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 02/04/2016 5 NDC: 61919-250-82 180 in 1 BOTTLE; Type 0: Not a Combination Product 02/04/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078181 02/04/2016 Labeler - DIRECT RX (079254320) Establishment Name Address ID/FEI Business Operations DIRECT RX 079254320 repack(61919-250)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.