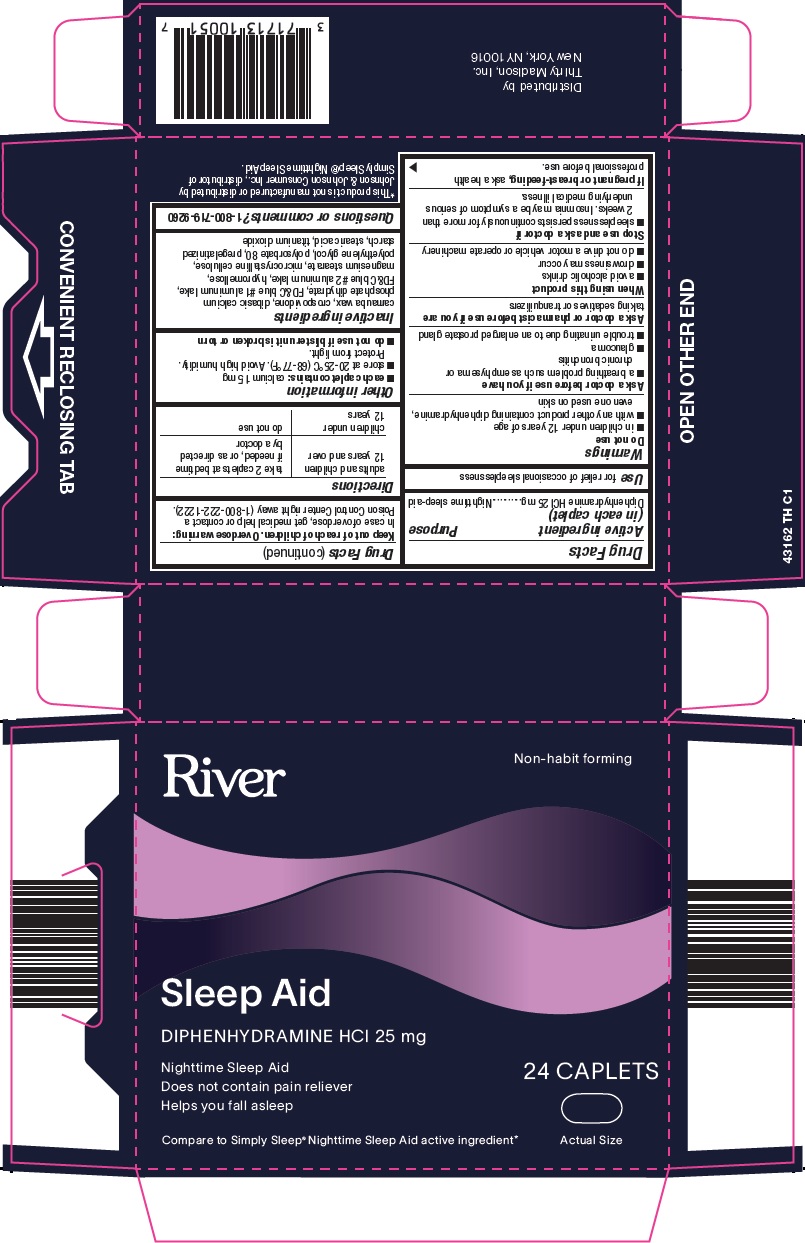
Thirty Madison Inc Sleep Aid Drug Facts
sleep aid by
Drug Labeling and Warnings
sleep aid by is a Otc medication manufactured, distributed, or labeled by THIRTY MADISON INC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SLEEP AID- diphenhydramine hydrochloride tablet, film coated
THIRTY MADISON INC
----------
Thirty Madison Inc Sleep Aid Drug Facts
Warnings
Do not use
- in children under 12 years of age
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- trouble urinating due to an enlarged prostate gland
- glaucoma
When using this product
- avoid alcoholic drinks
- drowsiness may occur
- do not drive a motor vehicle or operate machinery
Directions
|
adults and children 12 years and over |
take 2 caplets at bedtime if needed, or as directed by a doctor |
|
children under 12 years |
do not use |
Other information
- each caplet contains: calcium 15 mg
- store at 20-25°C (68-77°F). Avoid high humidity. Protect from light.
- do not use if blister unit is broken or torn
| SLEEP AID
diphenhydramine hydrochloride tablet, film coated |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - THIRTY MADISON INC (080774087) |
Revised: 7/2024
Document Id: d88dc5a3-ff10-4d0f-aaa1-439d6c557976
Set id: 2b5ac07f-7690-4ca4-95ee-d8369e69d104
Version: 2
Effective Time: 20240717
Trademark Results [sleep aid]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SLEEP AID 97858312 not registered Live/Pending |
Shenzhen Kaiwensi Electronic Commerce Co., Ltd. 2023-03-27 |
 SLEEP AID 97847521 not registered Live/Pending |
Shenzhen Kaiwensi Electronic Commerce Co., Ltd. 2023-03-20 |
 SLEEP AID 97765561 not registered Live/Pending |
Shenzhen Kaiwensi Electronic Commerce Co., Ltd. 2023-01-24 |
 SLEEP AID 88743606 not registered Live/Pending |
Plant Therapy LLC 2019-12-31 |
 SLEEP AID 78688138 3120450 Dead/Cancelled |
T. Harmon Inc. 2005-08-08 |
 SLEEP AID 77928191 not registered Dead/Abandoned |
EVEREST NUTRITION CORP 2010-02-04 |
 SLEEP AID 77448243 not registered Dead/Abandoned |
HEALCEUTICALS, LLC 2008-04-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.