LOSEASONIQUE- levonorgestrel/ethinyl estradiol and ethinyl estradiol kit
LoSeasonique by
Drug Labeling and Warnings
LoSeasonique by is a Prescription medication manufactured, distributed, or labeled by Teva Women's Health, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use LOSEASONIQUE tablets safely and effectively. See full prescribing information for LoSeasonique.
LOSEASONIQUE® (levonorgestrel/ethinyl estradiol tablets and ethinyl estradiol tablets) for oral use
Initial U.S. Approval: 1982WARNING: CIGARETTE SMOKING AND SERIOUS CARDIOVASCULAR EVENTS
See full prescribing information for complete boxed warning
- Women who are over 35 years old and smoke should not use LOSEASONIQUE. (4)
- Cigarette smoking increases the risk of serious cardiovascular events from combination oral contraceptive (COC) use.
RECENT MAJOR CHANGES
Warnings and Precautions (5.10) XX/XXXX
INDICATIONS AND USAGE
LOSEASONIQUE is an estrogen/progestin COC indicated for use by females to prevent pregnancy. (1)
DOSAGE AND ADMINISTRATION
Take one tablet daily by mouth at the same time every day for 91 days in the order directed on the blister pack. (2)
DOSAGE FORMS AND STRENGTHS
LOSEASONIQUE consists of 84 orange tablets containing 0.1 mg levonorgestrel and 20 mcg ethinyl estradiol, and 7 yellow tablets containing 10 mcg ethinyl estradiol. (3)
CONTRAINDICATIONS
- A high risk of arterial or venous thrombotic diseases (4)
- Undiagnosed abnormal uterine bleeding (4)
- Breast cancer or other estrogen-or progestin-sensitive cancer (4)
- Liver tumors or liver disease (4)
- Pregnancy (4)
- Co-administration with Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir (4)
WARNINGS AND PRECAUTIONS
- Vascular risks: Stop if a thromboticor thromboembolic event occurs. Stop at least 4 weeks before and through 2 weeks after major surgery. Start no earlier than 4 weeks after delivery, in women who are not breastfeeding. (5.1)
- Liver disease: Discontinue if jaundice occurs. (5.2)
- Hypertension:If used in females with well-controlled-hypertension, monitor blood pressure and stop use if blood pressure rises significantly. (5.3)
- Gallbladder disease: May cause or worsen gallbladder disease. (5.6)
- Carbohydrate and lipid metabolic effects: Monitor glucose in prediabetic and diabetic women taking LOSEASONIQUE. Consider an alternate contraceptive method for women with uncontrolled dyslipidemias. (5.7)
- Headache: Evaluate significant change in headaches and discontinue if indicated. (5.8)
- Uterine bleeding: May cause irregular bleeding or amenorrhea. Evaluate for other causes if symptoms persist. (5.9)
ADVERSE REACTIONS
The most common adverse reactions in clinical trials for LOSEASONIQUE were headaches, irregular and/or heavy uterine bleeding, nausea and/or vomiting, and back pain. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Teva Pharmaceuticals at 1-888-483-8279 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Enzyme inducers (e.g., CYP3A4): May decrease the effectiveness of LOSEASONIQUE or increase breakthrough bleeding. Counsel patients to use a back-up method or alternative method of contraception when enzyme inducers are used with LOSEASONIQUE. (7.1)
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: CIGARETTE SMOKING AND SERIOUS CARDIOVASCULAR EVENTS
RECENT MAJOR CHANGES
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 How to Start and Take LOSEASONIQUE
2.2 Dosing LOSEASONIQUE
2.3 Missed Doses
2.4 Advice in Case of Gastrointestinal Disturbances
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Thromboembolic and Other Vascular Conditions
5.2 Liver Disease
5.3 Hypertension
5.4 Risk of Liver Enzyme Elevations with Concomitant Hepatitis C Treatment
5.5 Age-related Considerations
5.6 Gallbladder Disease
5.7 Adverse Carbohydrate and Lipid Metabolic Effects
5.8 Headache
5.9 Bleeding Irregularities and Amenorrhea
5.10 Depression
5.11 Cervical Cancer
5.12 Effect on Binding Globulin
5.13 Hereditary Andioedema
5.14 Chloasma
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on Combined Oral Contraceptives
7.2 Effects of Combined Oral Contraceptives on Other Drugs
7.3 Concomitant Use with Hepatitis C Virus (HCV) Combination Therapy – Liver Enzyme Elevation
7.4 Effect on Laboratory Tests
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2. Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: CIGARETTE SMOKING AND SERIOUS CARDIOVASCULAR EVENTS
Cigarette smoking increases the risk of serious cardiovascular events from combination oral contraceptives (COC) use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked. For this reason, COCs, including LOSEASONIQUE, are contraindicated in women who are over 35 years of age and smoke. [See CONTRAINDICATIONS (4).]
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 How to Start and Take LOSEASONIQUE
Instruct the patient to begin taking LOSEASONIQUE on the first Sunday after the onset of menstruation. If menstruation begins on a Sunday, the first orange tablet is taken that day. One orange tablet should be taken daily for 84 consecutive days, followed by one yellow tablet for 7 consecutive days. A non-hormonal back-up method of contraception (such as condoms or spermicide) should be used until an orange tablet has been taken daily for 7 consecutive days. A scheduled period should occur during the 7 days that the yellow tablets are taken.
Begin the next and all subsequent 91-day cycles without interruption on the same day of the week (Sunday) on which the patient began her first dose of LOSEASONIQUE, following the same schedule: 84 days taking an orange tablet followed by 7 days taking a yellow tablet. If the patient does not immediately start her next pill pack, she should protect herself from pregnancy by using a non-hormonal back-up method of contraception until she has taken an orange tablet daily for 7 consecutive days.
For postpartum women who are not breastfeeding, start LOSEASONIQUE no earlier than four to six weeks postpartum due to increased risk of thromboembolism. If the patient starts on LOSEASONIQUE postpartum and has not yet had a period, evaluate for possible pregnancy, and instruct her to use an additional method of contraception until she has taken an orange tablet for 7 consecutive days.
2.2 Dosing LOSEASONIQUE
Instruct the patient to take one tablet by mouth at the same time every day. The dosage of LOSEASONIQUE is one orange tablet containing levonorgestrel and ethinyl estradiol daily for 84 consecutive days, followed by one yellow ethinyl estradiol tablet for 7 days. To achieve maximum contraceptive effectiveness, LOSEASONIQUE must be taken exactly as directed, in the order directed, and at intervals not exceeding 24 hours. The failure rate may increase when pills are missed or taken incorrectly.
If unscheduled spotting or bleeding occurs, instruct the patient to continue on the same regimen. If the bleeding is persistent or prolonged, advise the patient to consult her healthcare provider.
2.3 Missed Doses
Instruct patients about the handling of missed doses and to follow the dosing instructions provided in the FDA-approved Patient Labeling (Guide for Using LOSEASONIQUE).
Table 1. Instructions for Missed LOSEASONIQUE Tablets
If 1 orange tablet is missed
Take it as soon as possible. Take the next tablet at the regular time. Continue taking one tablet a day until the pack is finished. A back-up birth control method is not required if the patient has sex.
If 2 orange tablets in a row are missed
Take the two missed as soon as possible, and the next two active tablets the next day. Continue taking one tablet a day until the pack is finished. Additional nonhormonal contraception (such as condoms and spermicide) MUST be used if the patient has sex within 7 days after missing tablets.
If 3 or more orange tablets in a row are missed
Do not take the missed pills. Continue taking one tablet every day as indicated on the pack until the pack is finished. The patient may experience bleeding during the week following the missed pills. Additional nonhormonal contraception (such as condoms and spermicide) MUST be used if the patient has sex within 7 days after missing tablets.
If any of the 7 yellow tablets are missed
Throw away the missed tablets. Continue taking the remaining tablets until the pack is finished. A backup birth control method is not needed.
-
3 DOSAGE FORMS AND STRENGTHS
LOSEASONIQUE tablets are available in Extended-Cycle Tablet Dispensers, each containing a 13-week supply of tablets: 84 orange tablets, each containing 0.1 mg of levonorgestrel and 20 mcg ethinyl estradiol, and 7 yellow tablets each containing 10 mcg of ethinyl estradiol. The orange tablets are round, film-coated, unscored tablets with a debossed stylized b on one side and 28 on the other side. The yellow tablets are round, film-coated, unscored tablet with a debossed stylized b on one side and 556 on the other side.
-
4 CONTRAINDICATIONS
LOSEASONIQUE is contraindicated in females who are known to have the following conditions:
- A high risk of arterial or venous thrombotic diseases. Examples include females who are known to:
- Smoke, if over age 35 [see Boxed Warning and Warnings and Precautions (5.1)]
- Have current or history of deep vein thrombosis or pulmonary embolism [see Warnings and Precautions (5.1)]
- Have cerebrovascular disease [see Warnings and Precautions (5.1)]
- Have coronary artery disease [see Warnings and Precautions (5.1)]
- Have thrombogenic valvular or thrombogenic rhythm diseases of the heart (for example, subacute bacterial endocarditis with valvular disease, or atrial fibrillation) [see Warnings and Precautions (5.1)]
- Have inherited or acquired hypercoagulopathies [see Warnings and Precautions (5.1)]
- Have uncontrolled hypertension or hypertension with vascular disease [see Warnings and Precautions (5.1)]
- Have diabetes mellitus and are over age 35, diabetes mellitus with hypertension or with vascular disease or other end-organ damage, or diabetes mellitus of > 20 years duration [see Warnings and Precautions (5.7)]
- Have headaches with focal neurological symptoms, migraine headaches with aura, or over age 35 with any migraine headaches [see Warnings and Precautions (5.8)]
- Current or history of breast cancer or other estrogen- or progestin-sensitive cancer
- Liver tumors, acute viral hepatitis, or severe (decompensated) cirrhosis [see Warnings and Precautions (5.2)]
- Undiagnosed abnormal uterine bleeding [see Warnings and Precautions (5.8)]
- Pregnancy, because there is no reason to use COCs during pregnancy [see Warnings and Precautions (5.9) and Use in Specific Populations (8.1)]
- Use of Hepatitis C drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, due to the potential for ALT elevations [see Warnings and Precautions (5.4)].
- A high risk of arterial or venous thrombotic diseases. Examples include females who are known to:
-
5 WARNINGS AND PRECAUTIONS
5.1 Thromboembolic and Other Vascular Conditions
- Stop LOSEASONIQUE if an arterial or deep venous thromboembolic event occurs..
- Stop LOSEASONIQUE if there is unexplained loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions and evaluate for retinal vein thrombosis immediately.
- Discontinue LOSEASONIQUE during prolonged immobilization. If feasible, stop LOSEASONIQUE at least 4 weeks before and through 2 weeks after major surgery, or other surgeries known to have an elevated risk of thromboembolism.
- Start LOSEASONIQUE no earlier than 4 weeks after delivery, in females who are not breastfeeding. The risk of postpartum thromboembolism decreases after the third postpartum week, whereas the risk of ovulation increases after the third postpartum week.
- Before starting LOSEASONIQUE evaluate any past medical history or family history of thrombotic or thromboembolic disorders and consider whether the history suggests an inherited or acquired hypercoagulopathy. LOSEASONIQUE is contraindicated in females with a high risk of arterial or venous/thromboembolic diseases [see Contraindications (4)].
Arterial Events
COCs increase the risk of cardiovascular events and cerebrovascular events, such as myocardial infarction and stroke. The risk is greater among older women (> 35 years of age), smokers, and females with hypertension, dyslipidemia, diabetes, or obesity.
LOSEASONIQUE is contraindicated in women over 35 years of age who smoke [see Contraindications (4)]. Cigarette smoking increases the risk of serious cardiovascular events from COC use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked.
Venous Events
Use of COCs increases the risk of venous thromboembolic events (VTEs), such as deep vein thrombosis and pulmonary embolism. Risk factors for VTEs include smoking, obesity, and family history of VTE, in addition to other factors that contraindicate use of CHCs [see Contraindications (4)]. While the increased risk of VTE associated with use of COCs is well-established, the rates of VTE are even greater during pregnancy, and especially during the postpartum period (see Figure 1). The rate of VTE in females using COCs has been estimated to be 3 to 9 cases per 10,000 woman years.
The risk of VTE is highest during the first year of use of a COC and when restarting hormonal contraception after a break of four weeks or longer. Based on results from a few studies, there is some evidence that this is true for non-oral products as well. The risk of thromboembolic disease due to COCs gradually disappears after COC use is discontinued.
Figure 1 shows the risk of developing a VTE for females who are not pregnant and do not use oral contraceptives, for females who use oral contraceptives, and for females in the postpartum period. To put the risk of developing a VTE into perspective: If 10,000 females who are not pregnant and do not use oral contraceptives are followed for one year, between 1 and 5 of these females will develop a VTE.
Use of LOSEASONIQUE provides women with more hormonal exposure on a yearly basis than conventional monthly oral contraceptives containing the same strength synthetic estrogens and progestins (an additional 9 and 13 weeks of exposure to progestin and estrogen, respectively, per year).
5.2 Liver Disease
Elevated Liver Enzymes
LOSEASONIQUE is contraindicated in females with acute viral hepatitis or severe (decompensated) cirrhosis of the liver [see Contraindications (4)]. Discontinue LOSEASONIQUE if jaundice develops. Acute liver test abnormalities may necessitate the discontinuation of COC use until the liver tests return to normal and COC causation has been excluded.
Liver Tumors
LOSEASONIQUE is contraindicated in females with benign or malignant liver tumors [see Contraindications (4)]. COCs increase the risk of hepatic adenomas. An estimate of the attributable risk is 3.3 cases/100,000 COC users. Rupture of hepatic adenomas may cause death from abdominal hemorrhage.
Studies have shown an increased risk of developing hepatocellular carcinoma in long-term (> 8 years) COC users. The attributable risk of liver cancers in COC users is less than one case per million users.
5.3 Hypertension
LOSEASONIQUE is contraindicated in females with uncontrolled hypertension or hypertension with vascular disease [see Contraindications (4)]. For all females, including those with well-controlled hypertension, monitor blood pressure at routine visits and stop LOSEASONIQUE if blood pressure rises significantly.
An increase in blood pressure has been reported in females taking COCs, and this increase is more likely in older women and with extended duration of use. The effect of COCs on blood pressure may vary according to the progestin in the COC.
5.4 Risk of Liver Enzyme Elevations with Concomitant Hepatitis C Treatment
During clinical trials with the Hepatitis C combination drug regimen that contains obmitasvir/paritaprevir/ritonavir, with or without dasabuvir, ALT elevations greater than 5 times the upper limit of normal (ULN), including some cases greater than 20 times the ULN, were significantly more frequent in women using ethinyl estradiol-containing medications, such as COCs. Discontinue LOSEASONIQUE prior to starting therapy with the combination drug regimen ombitasvir/paritaprevir/ritonavir, with or without dasabuvir [see Contraindications (4)]. LOSEASONIQUE can be restarted approximately 2 weeks following completion of treatment with the Hepatitis C combination drug regimen.
5.5 Age-related Considerations
The risk for cardiovascular disease and prevalence of risk factors for cardiovascular disease increases with age. Certain conditions, such as smoking and migraine headache without aura, that do not contraindicate COC use in younger females, are contraindications to use in women over 35 years of age [see Contraindications (4) and Warnings and Precautions (5.1)]. Consider the presence of underlying risk factors that may increase the risk of cardiovascular disease or VTE, particularly before initiating a COC for women over 35 years, such as:
- Hypertension
- Diabetes
- Dyslipidemia
- Obesity
5.6 Gallbladder Disease
Studies suggest a small increased relative risk of developing gallbladder disease among COC users. Use of COCs may also worsen existing gallbladder disease.
A past history of COC-related cholestasis predicts an increased risk with subsequent COC use. Females with a history of pregnancy-related cholestasis may be at an increased risk for COC-related cholestasis.
5.7 Adverse Carbohydrate and Lipid Metabolic Effects
Hyperglycemia
LOSEASONIQUE is contraindicated in diabetic women over age 35, or females who have diabetes with hypertension, nephropathy, retinopathy, neuropathy, other vascular disease, or females with diabetes of > 20 years duration [see Contraindications (4)]. LOSEASONIQUE may decrease glucose tolerance. Carefully monitor prediabetic and diabetic females who are taking COCs.
Dyslipidemia
Consider alternative contraception for females with uncontrolled dyslipidemias. LOSEASONIQUE may cause adverse lipid changes.
Females with hypertriglyceridemia, or a family history thereof, may have an increase in serum triglyceride concentrations when using LOSEASONIQUE, which may increase the risk of pancreatitis.
5.8 Headache
LOSEASONIQUE is contraindicated in females who have headaches with focal neurological symptoms or have migraine headaches with aura, and in women over 35 years of age who have migraine headaches with or without aura [see Contraindications (4).
If a woman taking LOSEASONIQUE develops new headaches that are recurrent, persistent, or severe, evaluate the cause and discontinue LOSEASONIQUE if indicated. Consider discontinuation of LOSEASONIQUE if there is an increased frequency or severity of migraine during COC use (which may be prodromal of a cerebrovascular event).
5.9 Bleeding Irregularities and Amenorrhea
Unscheduled Bleeding and Spotting
Females using LOSEASONIQUE may experience unscheduled (breakthrough or intracyclic) bleeding and spotting, especially during the first 3 months of use. Bleeding irregularities may resolve over time or by changing to a different contraceptive product. If bleeding persists or occurs after previously regular cycles, evaluate for causes such as pregnancy or malignancy.
When prescribing LOSEASONIQUE, the convenience of fewer planned menses (4 per year instead of 13 per year) should be weighed against the inconvenience of increased unscheduled bleeding and/or spotting. The clinical trial that evaluated the efficacy of LOSEASONIQUE also assessed unscheduled bleeding. The participants in this 12-month clinical trial (N=2,185) completed the equivalent of over 20,000 28-day cycles of exposure and were composed primarily of women who had used OCs previously (89%), as opposed to new users (11%). A total of 209 subjects (9.6%) discontinued LOSEASONIQUE, at least in part, due to bleeding and/or spotting.
Scheduled (withdrawal) bleeding and/or spotting remained fairly constant over time, with an average of 2-3 days of bleeding and/or spotting per each 91-day cycle. Unscheduled bleeding and unscheduled spotting decreased over successive 91-day cycles. Table 2 below presents the number of days with unscheduled bleeding in treatment cycles 1 and 4. Table 3 presents the number of days with unscheduled spotting in treatment cycles 1 and 4.
Table 2: Total Number of Days with Unscheduled Bleeding 91-Day Treatment Cycle
Days per 84-Day Interval
Days per 28-Day Interval
Q1
Median
Q3
Mean
Mean
1st
0
5
11
7.5
2.5
4th
0
0
5
3.5
1.2
Q1=Quartile 1: 25% of women had this number of days of unscheduled bleeding
Median: 50% of women had ≤ this number of days of unscheduled bleeding
Q3=Quartile 3: 75% of women had ≤ this number of days of unscheduled bleeding
Table 3: Total Number of Days with Unscheduled Spotting 91-Day Treatment Cycle
Days per 84-Day Interval
Days per 28-Day Interval
Q1
Median
Q3
Mean
Mean
1st
3
10
19
14.0
4.7
4th
0
3
10
6.5
2.2
Q1=Quartile 1: 25% of women had ≤ this number of days of unscheduled spotting
Median: 50% of women had ≤ this number of days of unscheduled spotting
Q3=Quartile 3: 75% of women had ≤ this number of days of unscheduled spotting
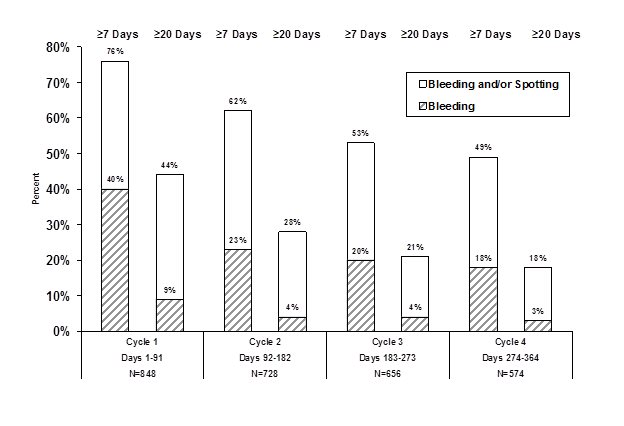
Figure 2 shows the percentage of LOSEASONIQUE subjects participating in the primary clinical trial with ≥7 days or ≥20 days of unscheduled bleeding and/or spotting, or just unscheduled bleeding, during each 91-day treatment cycle.
Figure 2: Percent of Women Taking LOSEASONIQUE who Reported Unscheduled Bleeding and/or Spotting (Based on Daily Diaries)
Amenorrhea and Oligomenorrhea
Females who use LOSEASONIQUE may experience absence of scheduled (withdrawal) bleeding, even if they are not pregnant.
If scheduled bleeding does not occur, consider the possibility of pregnancy.
After discontinuation of a COC, amenorrhea or oligomenorrhea may occur, especially if these conditions were pre-existent.
5.10 Depression
Carefully observe females with a history of depression and discontinue LOSEASONIQUE if depression recurs to a serious degree. Data on the association of COCs with the onset of depression or exacerbation of existing depression are limited.
5.11 Cervical Cancer
Some studies suggest that COCs are associated with an increase in the risk of cervical cancer or intraepithelial neoplasia. There is controversy about the extent to which these findings are due to differences in sexual behavior and other factors.
5.12 Effect on Binding Globulin
The estrogen component of LOSEASONIQUE may raise the serum concentrations of thyroxine-binding globulin, sex hormone-binding globulin, and cortisol-binding globulin. The dose of replacement thyroid hormone or cortisol therapy may need to be increased.
-
6 ADVERSE REACTIONS
The following serious adverse reactions with the use of COCs are discussed elsewhere in the labeling:
- Serious cardiovascular events [see Boxed Warning and Warnings and Precautions (5.1)]
- Vascular events [see Warnings and Precautions (5.1)]
- Liver disease [see Warnings and Precautions (5.2)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to the rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The clinical trial that evaluated the safety and efficacy of LOSEASONIQUE was a 12-month, multicenter, non-comparative open-label study, which enrolled women aged 18-41, of whom 2,185 took at least one dose of LOSEASONIQUE.
Adverse Reactions Leading to Study Discontinuation: 11% of the women discontinued from the clinical trial due to an adverse reaction; the most common adverse reactions leading to discontinuation were irregular and/or heavy uterine bleeding, headache, mood changes, nausea, acne, and weight gain.
Common Treatment-Emergent Adverse Reactions (≥5% of women): headaches (33%); irregular and/or heavy uterine bleeding (13%), dysmenorrhea (11%), nausea and/or vomiting (11%), back pain (8%).
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of LOSEASONIQUE. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency of establish a causal relationship to drug exposure.
-
7 DRUG INTERACTIONS
The sections below provide information on substances for which data on drug interactions with COCs are available. There is little information available about the clinical effect of most drug interactions that may affect COCs. However, based on the known pharmacokinetic effects of these drugs, clinical strategies to minimize any potential adverse effect on contraceptive effectiveness or safety are suggested.
Consult the approved product labeling of all concurrently used drugs to obtain further information about interactions with COCs or the potential for metabolic enzyme or transporter system alterations.
No formal drug-drug interaction studies were conducted with LOSEASONIQUE.
7.1 Effects of Other Drugs on Combined Oral Contraceptives
Substances Decreasing the Plasma Concentrations of COCs and Potentially Diminishing the Efficacy of COCs:
Table 4 includes substances that demonstrated an important drug interaction with LOSEASONIQUE.
Table 4: Significant Drug Interactions Involving Substances That Affect COCs
Metabolic Enzyme Inducers
Clinical effect
- Concomitant use of COCs with metabolic enzyme inducers may decrease the plasma concentrations of the estrogen and/or progestin component of COCs.
- Decreased exposure of the estrogen and/or progestin component of COCs may potentially diminish the effectiveness of COCs and may lead to contraceptive failure or an increase in breakthrough bleeding.
Prevention or management
- Counsel females to use an alternative method of contraception or a backup method when enzyme inducers are used with COCs.
- Continue backup contraception for 28 days after discontinuing the enzyme inducer to maintain contraceptive reliability.
Examples
Aprepitant, barbiturates, bosentan, carbamazepine, efavirenz, felbamate, griseofulvin, oxcarbazepine, phenytoin, rifampin, rifabutin, rufinamide, topiramate, products containing St. John’s worta, and certain protease inhibitors (see separate section on protease inhibitors below).
Colesevelam
Clinical effect
- Concomitant use of COCs with colesevelam significantly decreases systemic exposure of ethinyl estradiol.
- Decreased exposure of the estrogen component of COCs may potentially reduce contraceptive efficacy or result in an increase in breakthrough bleeding, depending on the strength of ethinyl estradiol in the COC.
Prevention or management
Administer 4 or more hours apart to attenuate this drug interaction.
a Induction potency of St. John’s wort may vary widely based on preparation.
Substances increasing the systemic exposure of COCs:
Co-administration of atorvastatin or rosuvastatin and COCs containing ethinyl estradiol increase systemic exposure of ethinyl estradiol by approximately 20 to 25 percent. Ascorbic acid and acetaminophen may increase systemic exposure of ethinyl estradiol, possibly by inhibition of conjugation. CYP3A inhibitors such as itraconazole, voriconazole, fluconazole, grapefruit juice,or ketoconazole may increase systemic exposure of the estrogen and/or progestin component of COCs.
Human immunodeficiency virus (HIV)/hepatitis C virus (HCV) protease inhibitors and non-nucleoside reverse transcriptase inhibitors:
Significant decreases in systemic exposure of the estrogen and/or progestin have been noted when COCs are co-administered with some HIV protease inhibitors (e.g., nelfinavir, ritonavir, darunavir/ritonavir, (fos)amprenavir/ritonavir, lopinavir/ritonavir, and tipranavir/ritonavir), some HCV protease inhibitors (e.g., boceprevir and telaprevir), and some non-nucleoside reverse transcriptase inhibitors (e.g., nevirapine).
In contrast, significant increases in systemic exposure of the estrogen and/or progestin have been noted when COCs are co-administered with certain other HIV protease inhibitors (e.g., indinavir and atazanavir/ritonavir) and with other non-nucleoside reverse transcriptase inhibitors (e.g., etravirine).
7.2 Effects of Combined Oral Contraceptives on Other Drugs
Table 5 provides significant drug interaction information for drugs co-administered with LOSEASONIQUE.
Table 5: Significant Drug Interaction Information for Drugs Co-Administered With COCs
Lamotrigine
Clinical effect
- Concomitant use of COCs with lamotrigine may significantly decrease systemic exposure of lamotrigine due to induction of lamotrigine glucuronidation.
- Decreased systemic exposure of lamotrigine may reduce seizure control.
Prevention or management
Dose adjustment may be necessary. Consult the approved product labeling for lamotrigine.
Thyroid Hormone Replacement Therapy or Corticosteroid Replacement Therapy
Clinical effect
Concomitant use of COCs with thyroid hormone replacement therapy or corticosteroid replacement therapy may increase systemic exposure of thyroid-binding and cortisol-binding globulin [see Warnings and Precautions (5.12)].
Prevention or management
The dose of replacement thyroid hormone or cortisol therapy may need to be increased. Consult the approved product labeling for the therapy in use [see Warnings and Precautions (5.12)].
Other Drugs
Clinical effect
Concomitant use of COCs may decrease systemic exposure of acetaminophen, morphine, salicylic acid, and temazepam. Concomitant use with ethinyl estradiol-containing COCs may increase systemic exposure of other drugs (e.g., cyclosporine, prednisolone, theophylline, tizanidine, and voriconazole).
Prevention or management
The dosage of drugs that can be affected by this interaction may need to be increased. Consult the approved product labeling for the concomitantly used drug.
7.3 Concomitant Use with Hepatitis C Virus (HCV) Combination Therapy – Liver Enzyme Elevation
Do not co-administer LOSEASONIQUE with HCV drug combinations containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir, due to potential for ALT elevations [see Warnings and Precautions (5.4)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
LOSEASONIQUE is contraindicated in pregnancy because there is no reason to use COCs in pregnancy. Discontinue LOSEASONIQUE if pregnancy occurs. Epidemiologic studies and meta-analyses have not found an increased risk of genital or non-genital birth defects (including cardiac anomalies and limb reduction defects) following exposure to COCs before conception or during early pregnancy.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies in 2 to 4 percent and 15 to 20 percent, respectively.
8.2. Lactation
Risk Summary
Contraceptive hormones and/or metabolites are present in human milk. COCs can reduce milk production in breastfeeding females. This reduction can occur at any time but is less likely to occur once breastfeeding is well-established. When possible, advise the nursing female to use other methods of contraception until she discontinues breastfeeding [See Dosage and Administration (2.2)].The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for LOSEASONIQUE and any potential adverse effects on the breastfed child from LOSEASONIQUE or the underlying maternal condition.
- 10 OVERDOSAGE
-
11 DESCRIPTION
LOSEASONIQUE (levonorgestrel/ethinyl estradiol and ethinyl estradiol) tablets provide an oral contraceptive regimen of 84 orange tablets each containing 0.1 mg levonorgestrel and 20 mcg ethinyl estradiol, followed by 7 yellow tablets each containing 10 mcg ethinyl estradiol.
The structural formulas for the active components are:
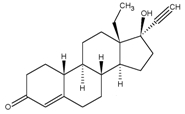
Levonorgestrel
C21H28O2 MW: 312.4
Levonorgestrel is chemically 18,19-Dinorpregn-4-en-20-yn-3-one, 13-ethyl-17-hydroxy-, (17α)-, (-)-.
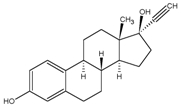
Ethinyl Estradiol
C20H24O2 MW: 296.4
Ethinyl Estradiol is 19-Norpregna-1,3,5(10)-trien-20-yne-3,17-diol, (17α)-.
Inactive ingredients for the orange tablets include FD&C Yellow # 6 (Sunset Yellow) aluminum lake, hypromellose, lactose, magnesium stearate, microcrystalline cellulose, corn starch, titanium dioxide and triacetin.
Inactive ingredients for the yellow tablets include anhydrous lactose, FD&C Yellow # 10 aluminum lake, FD&C Yellow # 6 (Sunset Yellow) aluminum lake, hypromellose, magnesium stearate, microcrystalline cellulose, polacrilin potassium, polyethylene glycol, polysorbate 80 and titanium dioxide.
-
12 CLINICAL PHARMACOLOGY
12.3 Pharmacokinetics
Absorption
No specific investigation of the absolute bioavailability of LOSEASONIQUE in humans has been conducted. However, literature indicates that levonorgestrel is rapidly and completely absorbed after oral administration (bioavailability nearly 100%) and is not subject to first-pass metabolism. Ethinyl estradiol is rapidly and almost completely absorbed from the gastrointestinal tract but, due to first-pass metabolism in gut mucosa and liver, the systemic bioavailability of ethinyl estradiol is approximately 43%.
The mean plasma pharmacokinetic parameters of LOSEASONIQUE following a single oral dose of three levonorgestrel/ethinyl estradiol combination tablets in normal healthy women under fasting conditions are reported in Table 6.
Table 6: Mean (SD) Pharmacokinetic Parameters Following a Single Dose Administration of Three Tablets of LOSEASONIQUE in 30 Healthy Women under Fasting Conditions AUC0-∞
Cmax
Tmax
T½
Levonorgestrel
76.5 ± 24.9 ng*hr/mL
6.0 ± 1.6 ng/mL
1.6 ± 0.6 hours
28.5 ± 8.7 hours
Ethinyl estradiol
1335.8 ± 365.3 pg*hr/mL
122.8 ± 39.5 pg/mL
1.8 ± 0.7 hours
17.5 ± 7.4 hours
AUC0-∞ = area under the drug concentration curve from time 0 to infinity
Cmax = maximum concentration
Tmax = time to maximum concentration
The effect of food on the rate and the extent of levonorgestrel and ethinyl estradiol absorption following oral administration of LOSEASONIQUE has not been evaluated.
Distribution
The apparent volume of distribution of levonorgestrel and ethinyl estradiol is reported to be approximately 1.8 L/kg and 4.3 L/kg, respectively. Levonorgestrel is about 97.5 to 99% protein-bound, principally to sex hormone binding globulin (SHBG) and, to a lesser extent, serum albumin. Ethinyl estradiol is about 95 to 97% bound to serum albumin. Ethinyl estradiol does not bind to SHBG, but induces SHBG synthesis, which leads to decreased levonorgestrel clearance. Following repeated daily dosing of combination levonorgestrel/ethinyl estradiol OCs, levonorgestrel plasma concentrations accumulate more than predicted based on single-dose pharmacokinetics, due in part, to increased SHBG levels that are induced by ethinyl estradiol, and a possible reduction in hepatic metabolic capacity.
Metabolism
Following absorption, levonorgestrel is conjugated at the 17β-OH position to form sulfate conjugates and, to a lesser extent, glucuronide conjugates in plasma. Significant amounts of conjugated and unconjugated 3α, 5β-tetrahydrolevonorgestrel are also present in plasma, along with much smaller amounts of 3α, 5α-tetrahydrolevonorgestrel and 16β-hydroxylevonorgestrel. Levonorgestrel and its phase I metabolites are excreted primarily as glucuronide conjugates. Metabolic clearance rates may differ among individuals by several-fold, and this may account in part for the wide variation observed in levonorgestrel concentrations among users.
First-pass metabolism of ethinyl estradiol involves formation of ethinyl estradiol-3-sulfate in the gut wall, followed by 2-hydroxylation of a portion of the remaining untransformed ethinyl estradiol by hepatic cytochrome P-450 3A4 (CYP3A4). Levels of CYP3A4 vary widely among individuals and can explain the variation in rates of ethinyl estradiol hydroxylation. Hydroxylation at the 4-, 6-, and 16- positions may also occur, although to a much lesser extent than 2-hydroxylation. The various hydroxylated metabolites are subject to further methylation and/or conjugation.
Excretion
About 45% of levonorgestrel and its metabolites are excreted in the urine and about 32% are excreted in feces, mostly as glucuronide conjugates. Ethinyl estradiol is excreted in the urine and feces as glucuronide and sulfate conjugates, and then undergoes enterohepatic recirculation.
Race
The effect of race on the pharmacokinetics of LOSEASONIQUE has not been evaluated.
Renal and Hepatic Impairment
No formal studies were conducted to evaluate the effect of hepatic or renal disease on the disposition of LOSEASONIQUE. However, steroid hormones may be poorly metabolized in patients with impaired liver function.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
In a 12-month multicenter open-label clinical trial, 2,185 women aged 18-41 were studied to assess the safety and efficacy of LOSEASONIQUE, completing the equivalent of 20,937 28-day cycles of exposure. The racial demographic of those enrolled was: Caucasian (75%), African-American (12%), Hispanic (10%), Asian (2%), and Other (2%). There were no exclusions for body mass index (BMI) or weight. The weight range for those women treated was 87 to 381 lbs., with a mean weight of 159 lbs. Among the women in the trial, 59% were current or recent hormonal contraceptive users, 30% were prior users (had used hormonal contraceptives in the past but not in the 6 months prior to enrollment) and 11% were new starts. Of treated women, 14.2% were lost to follow-up, 11.6% discontinued due to an adverse event, and 10.3% discontinued by withdrawing their consent.
The pregnancy rate (Pearl Index [PI]) in women aged 18 to 35 years was 2.74 pregnancies per 100 women-years of use (95% confidence interval 1.92 – 3.78), based on 36 pregnancies that occurred after the onset of treatment and within 14 days after the last combination pill. Cycles in which conception did not occur, but which included the use of backup contraception, were not included in the calculation of the PI. The PI includes patients who did not take the drug correctly.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
LOSEASONIQUE (levonorgestrel/ethinyl estradiol tablets and ethinyl estradiol tablets) are available in an Extended-Cycle Tablet Dispenser that contains 84 round, orange tablets and 7 round, yellow tablets. Each orange tablet (debossed stylized b on one side and 28 on the other side) contains 0.1 mg levonorgestrel and 20 mcg ethinyl estradiol. Each yellow tablet (debossed stylized b on one side and 556 on the other side) contains 10 mcg ethinyl estradiol.
Box of 2 Extended-Cycle Tablet Dispensers NDC: 51285-092-87
16.2 Storage and Handling
Store at 20° to 25°C (68° to77°F) [See USP Controlled Room Temperature].
The tablets should not be removed from the protective blister packaging and outer plastic dispenser to avoid damage to the product. The plastic dispenser should be kept in the foil pouch until dispensed to the patient.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved Patient Labeling (Guide for Using LOSEASONIQUE).
Counsel patients on the following information:
- Cigarette smoking increases the risk of serious cardiovascular events from COC use.Women who are over 35 years old and smoke should not use LOSEASONIQUE [see Boxed Warning and Warnings and Precautions (5.1)].
- The increased risk of VTE compared to non-users of COCs is greatest after initially starting a COC or restarting (following a 4-week or greater interruption in intake) the same or a different COC [see Warnings and Precautions (5.1)].
- LOSEASONIQUE is not to be used during pregnancy. Instruct the patient to stop further intake of LOSEASONIQUE if pregnancy is confirmed during treatment [see Contraindications (4)].
- LOSEASONIQUE does not protect against HIV-infectionand other sexually transmitted infections.
- Patients should take one tablet daily by mouth at the same time every day. Instruct patients what to do in the event pills are missed [see Dosage and Administration (2.3)].
- Need for Additional Contraception
- Postpartum females who have not yet had a period when they start LOSEASONIQUE need to use an additional method of contraception until they have taken an orange tablet for 7 consecutive days [see Dosage and Administration (2.2)].
- There is a need for a back-up or alternative method of contraception when enzyme inducers are used with LOSEASONIQUE [see Drug Interactions (7.1)].
- LOSEASONIQUE may reduce breast milk production. This is less likely to occur if breastfeeding is well established. When possible, nursing women should use other methods of contraception until they have discontinued breastfeeding [see Use in Specific Populations (8.2)].
- Amenorrhea may occur. Advise the patient to contact a healthcare provider in the event of amenorrhea with symptoms of pregnancy, such as morning sickness or unusual breast tenderness.
TEVA WOMEN’S HEALTH, INC.
Subsidiary of TEVA PHARMACEUTICALS USA, INC.
North Wales, PA 19454LOSEAQ-001
-
FDA-approved Patient Labeling
Guide for Using LoSeasonique®
WARNING TO WOMEN WHO SMOKE
Do not use LOSEASONIQUE if you smoke cigarettes and are over 35 years old. Smoking increases your risk of serious cardiovascular side effects from birth control pills, including death from heart attack, blood clots or stroke. This risk increases with age and the number of cigarettes you smoke.
Birth control pills help to lower the chances of becoming pregnant. They do not protect against HIV infection (AIDS) and other sexually transmitted infections.
WHAT IS LOSEASONIQUE?
LOSEASONIQUE is a birth control pill. It contains two female hormones, an estrogen called ethinyl estradiol, and a progestin called levonorgestrel.
HOW WELL DOES LOSEASONIQUE WORK?
Your chance of getting pregnant depends on how well you follow the directions for taking your birth control pills. The more carefully you follow the directions, the less chance you have of getting pregnant.
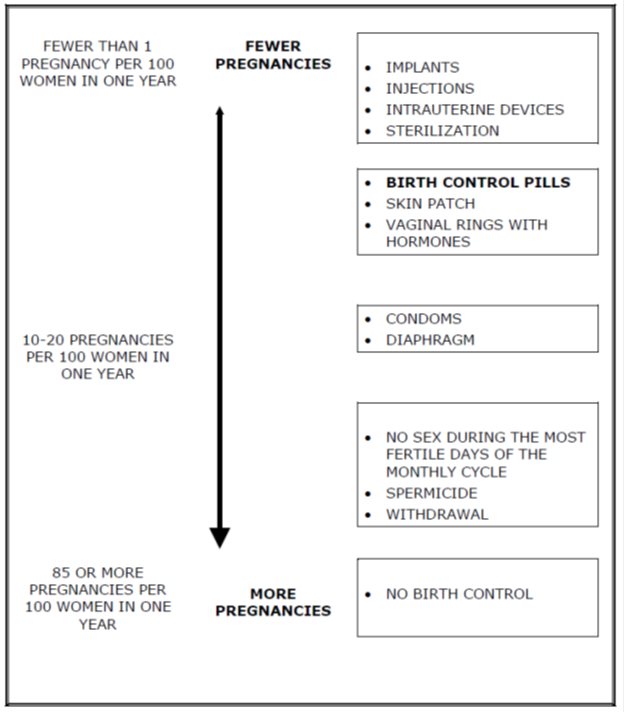
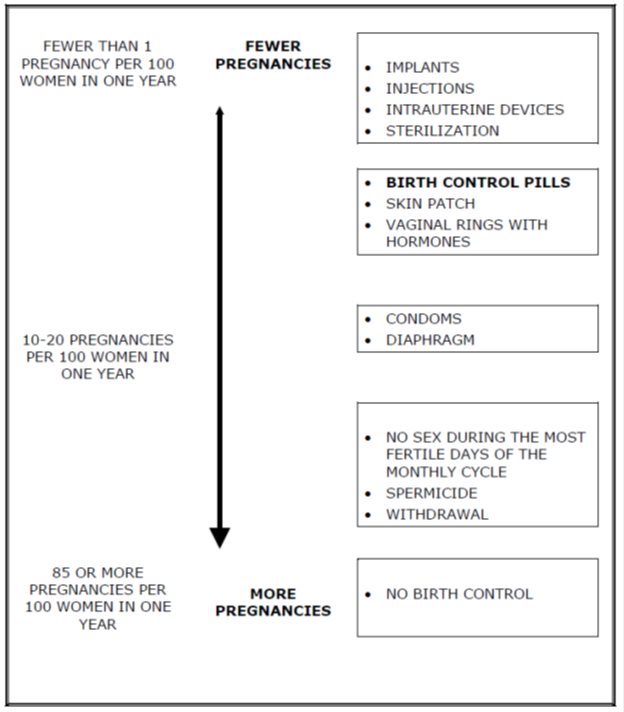
Based on the results of a single clinical study lasting 12 months, 2 to 4 women, out of 100 women, may get pregnant during the first year they use LOSEASONIQUE.
The following chart shows the chance of getting pregnant for women who use different methods of birth control. Each box on the chart contains a list of birth control methods that are similar in effectiveness. The most effective methods are at the top of the chart. The box on the bottom of the chart shows the chance of getting pregnant for women who do not use birth control and are trying to get pregnant.
HOW DO I TAKE Loseasonique?
- Take one pill every day at the same time. If you miss pills you could get pregnant. This includes starting the pack late. The more pills you miss, the more likely you are to get pregnant.
- Many women have spotting or light bleeding, or may feel sick to their stomach during the first few months of taking LOSEASONIQUE. If you feel sick to your stomach, do not stop taking the pill. The problem will usually go away. If it doesn't go away, check with your healthcare provider.
- Missing pills can also cause spotting or light bleeding, even when you take the missed pills later. On the days you take 2 pills to make up for missed pills, you could also feel a little sick to your stomach.
- If you have trouble remembering to take LOSEASONIQUE, talk to your healthcare provider about how to make pill-taking easier or about using another method of birth control.
Before you start taking LOSEASONIQUE
- Decide what time of day you want to take your pill. It is important to take it at about the same time every day.
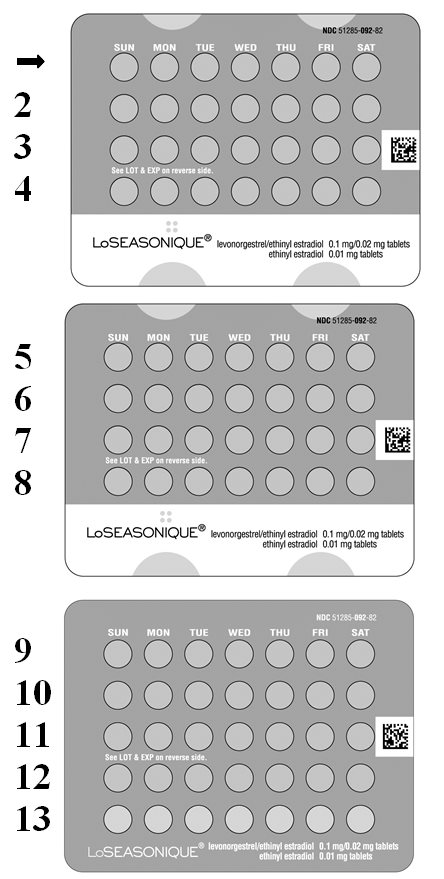
- Look at your Extended-Cycle Tablet Dispenser. Your Tablet Dispenser consists of 3 trays with cards that hold 91 individually sealed pills (a 13-week or 91-day cycle). The 91 pills consist of 84 orange and 7 yellow pills. Trays 1 and 2 each contain 28 orange pills (4 rows of 7 pills). Tray 3 contains 35 pills consisting of 28 orange pills (4 rows of 7 pills) and 7 yellow pills (1 row of 7 pills).
- Also find:
- Where on the first tray in the pack to start taking pills (upper left corner at the start arrow) and
- In what order to take the pills (follow the weeks and arrow).
- Be sure you have ready at all times another kind of birth control (such as condoms or spermicides), to use as a back-up in case you miss pills.
When to Start LOSEASONIQUE
- Take the first orange pill on the Sunday after your period starts, even if you are still bleeding. If your period begins on Sunday, start the first orange pill that same day.
- Use another method of birth control (such as condoms or spermicides) as a back-up method if you have sex anytime from the Sunday you start your first orange pill until the next Sunday (first 7 days). If you have been using a different hormonal method of birth control (such as a different pill, the “patch,” or the “vaginal ring”), you need to use another method of birth control (such as condoms or spermicides) each time you have sex after stopping your old method of birth control until you have taken LOSEASONIQUE for 7 days.
How to Take LOSEASONIQUE
- Take one pill at the same time every day until you have taken the last pill in the tablet dispenser.
- Do not skip pills even if you are experiencing spotting or bleeding or feel sick to your stomach (nausea).
- Do not skip pills even if you do not have sex very often.
- When you finish a tablet dispenser
- After taking the last yellow pill, start taking the first orange pill from a new Extended-Cycle Tablet Dispenser the very next day (this should be on a Sunday) regardless of when your period started.
- If you miss your scheduled period when you are taking the yellow pills, contact your healthcare provider because you may be pregnant. If you are pregnant, you should stop taking LOSEASONIQUE.
WHAT TO DO IF YOU MISS PILLS
If you MISS 1 orange pill:
- Take it as soon as you remember. Take the next pill at your regular time. This means you may take 2 pills in 1 day.
- You do not need to use a back-up birth control method if you have sex.
If you MISS 2 orange pills in a row:
- Take 2 pills on the day you remember, and 2 pills the next day.
- Then take 1 pill a day until you finish the pack.
- You could become pregnant if you have sex in the 7 days after you miss two pills. You MUST use another birth control method (such as condoms or spermicide) as a back up for the 7 days after you restart your pills.
If you MISS 3 OR MORE orange pills in a row:
- Do not take the missed pills. Keep taking 1 pill every day as indicated on the pack until you have completed all of the remaining pills in the pack. For example: If you resume taking the pill on Thursday, take the pill under “Thursday” and do not take the missed pills. You may experience bleeding during the week following the missed pills.
- You could become pregnant if you have sex during the days of missed pills or during the first 7 days after restarting your pills.
- You MUST use a non-hormonal birth control method (such as condoms or spermicide) as a back-up when you miss pills and for the first 7 days after you restart your pills. If you do not have your period when you are taking the yellow pills, call your healthcare provider because you may be pregnant.
If you MISS ANY of the 7 yellow pills:
- Throw away the missed pills.
- Keep taking the scheduled pills until the pack is finished.
- You do not need a back-up method of birth control.
Finally, if you are still not sure what to do about the pills you have missed
- Use a back-up method anytime you have sex.
- Keep taking one pill each day until you contact your healthcare provider.
WHO SHOULD NOT TAKE LOSEASONIQUE?
Your healthcare provider will not give you LOSEASONIQUE if you have:
- Ever had breast cancer or any cancer that is sensitive to female hormones
- Liver disease, including liver tumors
- Been prescribed any Hepatitis C drug combination containing ombitasvir/paritaprevir/ritonavir, with or without dasabuvir. This may increase levels of the liver enzyme “alanine aminotransferase” (ALT) in the blood.
- Ever had blood clots in your arms, legs, or lungs
- Ever had a stroke
- Ever had a heart attack
- Certain heart valve problems or heart rhythm abnormalities that can cause blood clots to form in the heart
- An inherited problem with your blood that makes it clot more than normal
- High blood pressure that medicine can't control
- Diabetes
- with high blood pressure
- with kidney, eye, or blood vessel damage
- for more than 20 years
- Certain kinds of severe migraine headaches with aura, numbness, weakness or changes in vision
Also, do not take birth control pills if you:
- are over 35 years old and
- smoke
- have migraine headaches
- have diabetes
- are pregnant
Birth control pills may not be a good choice for you if you have ever had jaundice (yellowing of the skin or eyes) caused by pregnancy.
WHAT ELSE SHOULD I KNOW ABOUT TAKING LOSEASONIQUE?
Birth control pills do not protect you against any sexually transmitted disease, including HIV, the virus that causes AIDS.
Do not skip any pills, even if you do not have sex often.
Birth control pills should not be taken during pregnancy. However, birth control pills taken by accident during pregnancy are not known to cause birth defects.
You should stop LOSEASONIQUE at least four weeks before you have major surgery and not restart it for at least two weeks after the surgery, due to an increased risk of blood clots.
If you are breastfeeding, consider another birth control method until you are ready to stop breastfeeding. Birth control pills that contain estrogen, like LOSEASONIQUE, may decrease the amount of milk you make. A small amount of the pill's hormones pass into breast milk, but this has not caused harmful effects in breastfeeding infants.
Tell your health care provider about all medicines and herbal products that you take. Some medicines and herbal products may make birth control pills less effective, including:
- barbiturates
- bosentan
- carbamazepine
- felbamate
- griseofulvin
- oxcarbazepine
- phenytoin
- rifampin
- St. John’s wort
- topiramate
Consider using another birth control method when you take medicines that may make birth control pills less effective.
Birth control pills may interact with lamotrigine, an anticonvulsant used for epilepsy. This may increase the risk of seizures, so your physician may need to adjust the dose of lamotrigine.
If you have vomiting or diarrhea, your birth control pills may not work as well. Use another birth control method, like condoms or a spermicide, until you check with your health care provider.
WHAT ARE THE MOST SERIOUS RISKS OF TAKING BIRTH CONTROL PILLS?
Like pregnancy, birth control pills increase the risk of serious blood clots, especially in women who have other risk factors, such as smoking, obesity, or age >35. It is possible to die from a problem caused by a blood clot, such as a heart attack or a stroke. Some examples of serious blood clots are blood clots in the:
- Legs (thrombophlebitis)
- Lungs (pulmonary embolus)
- Eyes (loss of eyesight)
- Heart (heart attack)
- Brain (stroke)
A few women who take birth control pills may get:
- High blood pressure
- Gallbladder problems
- Rare cancerous or noncancerous liver tumors
All of these events are uncommon in healthy women.
Call your health care provider right away if you have:
- Persistent leg pain
- Sudden shortness of breath
- Sudden blindness, partial or complete
- Severe pain in your chest
- Sudden, severe headache unlike your usual headaches
- Weakness or numbness in an arm or leg, or trouble speaking
- Yellowing of the skin or eyeballs
WHAT ARE COMMON SIDE EFFECTS OF BIRTH CONTROL PILLS?
The most common side effects of birth control pills are:
- Spotting or bleeding between menstrual periods
- Nausea
- Breast tenderness
- Headache
These side effects are usually mild and usually disappear with time.
Less common side effects are:
- Acne
- Less sexual desire
- Bloating or fluid retention
- Blotchy darkening of the skin, especially on the face
- High blood sugar, especially in women who already have diabetes
- High fat levels in the blood.
- Depression, especially if you have had depression in the past. Call your health care provider immediately if you have any thoughts of harming yourself.
- Problems tolerating contact lenses
- Weight changes
This is not a complete list of possible side effects. Talk to your health care provider if you develop any side effects that concern you. You may report side effects to the FDA at 1‑800-FDA-1088.
No serious problems have been reported from a birth control pill overdose, even when accidentally taken by children.
DO BIRTH CONTROL PILLS CAUSE CANCER?
Birth control pills do not appear to cause breast cancer. However, if you have breast cancer now, or have had it in the past, do not use birth control pills because some breast cancers are sensitive to hormones.
Women who use birth control pills may have a slightly higher chance of getting cervical cancer. However, this may be due to other reasons such as having more sexual partners.
WHAT SHOULD I KNOW ABOUT MY PERIOD WHEN TAKING LOSEASONIQUE?
When you take LOSEASONIQUE, which has a 91-day extended dosing cycle, you should expect to have 4 scheduled periods per year (bleeding when you are taking the 7 yellow pills). Each period is likely to last about 2 to 3 days. However, you will probably have more bleeding or spotting between your scheduled periods than if you were using a birth control pill with a 28-day dosing cycle. This bleeding or spotting tends to decrease with time. Do not stop taking LOSEASONIQUE because of this bleeding or spotting. If the spotting continues for more than 7 consecutive days or if the bleeding is heavy, call your healthcare provider.
WHAT IF I MISS MY SCHEDULED PERIOD WHEN TAKING LOSEASONIQUE?
You should consider the possibility that you are pregnant if you miss your scheduled period (no bleeding on the days that you are taking yellow tablets). Since scheduled periods are less frequent when you are taking LOSEASONIQUE, notify your healthcare provider that you have missed your period and that you are taking LOSEASONIQUE. Also notify your healthcare provider if you have symptoms of pregnancy such as morning sickness or unusual breast tenderness. It is important that your healthcare provider evaluates you to determine if you are pregnant. Stop taking LOSEASONIQUE if it is determined that you are pregnant.
WHAT IF I WANT TO BECOME PREGNANT?
You may stop taking the pill whenever you wish. Consider a visit with your health care provider for a pre-pregnancy checkup before you stop taking the pill.
GENERAL ADVICE ABOUT LOSEASONIQUE
Your healthcare provider prescribed LOSEASONIQUE for you. Do not share LOSEASONIQUE with anyone else. Keep LOSEASONIQUE out of the reach of children.
If you have concerns or questions, ask your healthcare provider. You may also ask your healthcare providers for a more detailed label written for medical professionals.
Rx only
TEVA WOMEN’S HEALTH, INC.
Subsidiary of TEVA PHARMACEUTICALS USA, INC.
North Wales, PA 19454LOSEAQPL-001
Revised: XX/20XX
-
Package/Label Display Panel, Part 1 of 2
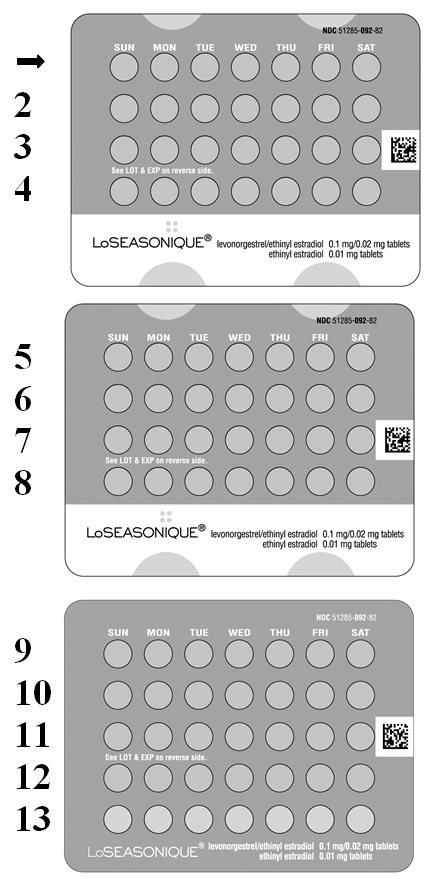
2 Extended-Cycle Tablet Dispensers
91 Tablets Each / NDC 51285-092-87
LoSEASONIQUE® levonorgestrel/ethinyl estradiol 0.1 mg/0.02 mg tablets ethinyl estradiol 0.01 mg tablets
Rx only
Contains 2 Extended-Cycle Tablet Dispensers, each containing 91 tablets: 84 orange tablets, each containing 0.1 mg levonorgestrel
with 0.02 mg ethinyl estradiol, and 7 yellow tablets, each containing 0.01 mg ethinyl estradiol.
TEVA
TEVA WOMEN’S HEALTH, INC.
Subsidiary of TEVA PHARMACEUTICALS USA, INC.
North Wales, PA 19454
- Package/Label Display Panel, Part 2 of 2
-
INGREDIENTS AND APPEARANCE
LOSEASONIQUE
levonorgestrel/ethinyl estradiol and ethinyl estradiol kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 51285-092 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51285-092-87 2 in 1 CARTON 03/23/2009 1 NDC: 51285-092-82 1 in 1 POUCH 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 84 Part 2 7 Part 1 of 2 LEVONORGESTREL/ETHINYL ESTRADIOL
levonorgestrel/ethinyl estradiol tablet, film coatedProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVONORGESTREL (UNII: 5W7SIA7YZW) (LEVONORGESTREL - UNII:5W7SIA7YZW) LEVONORGESTREL 0.1 mg ETHINYL ESTRADIOL (UNII: 423D2T571U) (ETHINYL ESTRADIOL - UNII:423D2T571U) ETHINYL ESTRADIOL 0.02 mg Inactive Ingredients Ingredient Name Strength FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYPROMELLOSE 2208 (3 MPA.S) (UNII: 9H4L916OBU) HYPROMELLOSE 2910 (15 MPA.S) (UNII: 36SFW2JZ0W) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color ORANGE Score no score Shape ROUND Size 6mm Flavor Imprint Code b;28 Contains 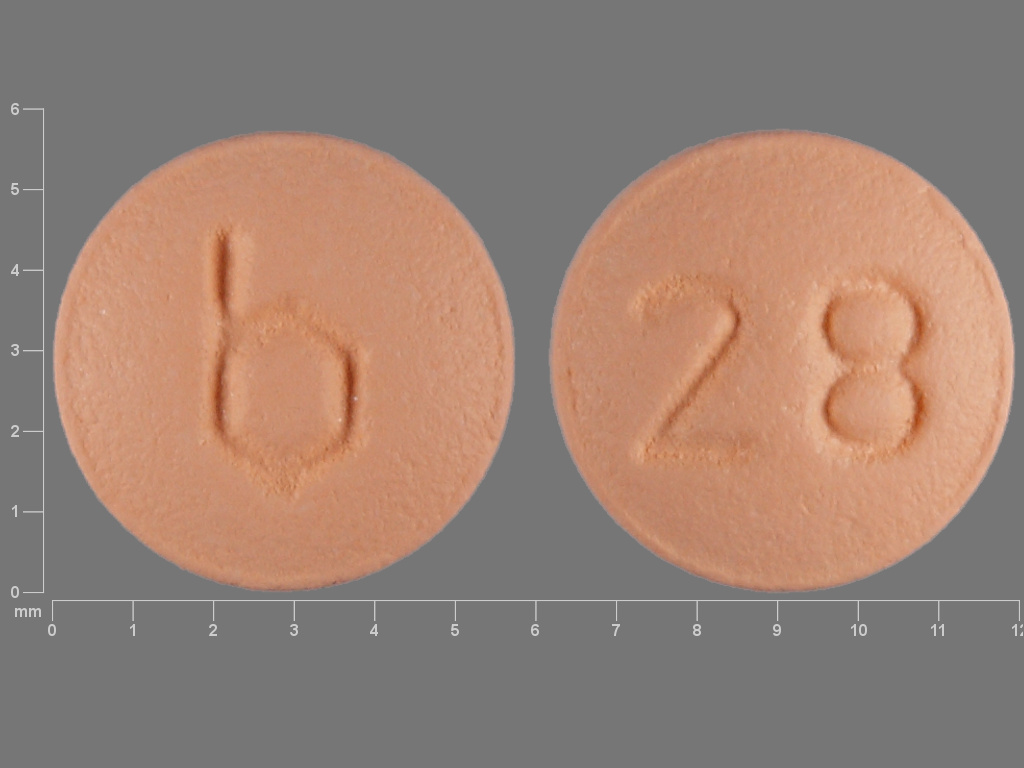
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022262 03/23/2009 Part 2 of 2 ETHINYL ESTRADIOL
ethinyl estradiol tablet, film coatedProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ETHINYL ESTRADIOL (UNII: 423D2T571U) (ETHINYL ESTRADIOL - UNII:423D2T571U) ETHINYL ESTRADIOL 0.01 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYPROMELLOSE 2910 (3 MPA.S) (UNII: 0VUT3PMY82) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLACRILIN POTASSIUM (UNII: 0BZ5A00FQU) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYSORBATE 80 (UNII: 6OZP39ZG8H) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color YELLOW Score no score Shape ROUND Size 6mm Flavor Imprint Code b;556 Contains 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022262 03/23/2009 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022262 03/23/2009 Labeler - Teva Women's Health, Inc. (017038951)
Trademark Results [LoSeasonique]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 LOSEASONIQUE 77969935 4035754 Live/Registered |
TEVA WOMEN'S HEALTH, LLC 2010-03-26 |
 LOSEASONIQUE 77340250 3636124 Live/Registered |
TEVA WOMEN'S HEALTH, LLC 2007-11-29 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
