SODIUM FLUORIDE F 18- sodium fluoride f-18 injection
Sodium Fluoride F 18 by
Drug Labeling and Warnings
Sodium Fluoride F 18 by is a Prescription medication manufactured, distributed, or labeled by SOFIE Co., N-Molecular, Inc. dba SOFIE, SOFIE Co. dba SOFIE. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Sodium Fluoride F 18 Injection, USP safely and effectively. See full prescribing information for Sodium Fluoride F 18 Injection, USP.
SODIUM FLUORIDE F 18 INJECTION, USP
For Intravenous Use
Initial U.S. Approval: XXXXINDICATIONS AND USAGE
Sodium Fluoride F 18 Injection, USP is a radioactive diagnostic agent for positron emission tomography (PET) indicated for imaging of bone to define areas of altered osteogenic activity(1).
DOSAGE AND ADMINISTRATION
- Sodium Fluoride F18 Injection emits radiation and must be handled with appropriate safety measures(2.1).
- Administer 300-450 MBq (8 to 12 mCi) as an intravenous injection in adults(2.4).
- Administer approximately 2.1 MBq/kg in children with a minimum of 19 MBq (0.5 mCi) and a maximum of 148 MBq (4 mCi) as an intravenous injection(2.5).
- Imaging can begin 1–2 hours after administration; optimally at one hour post administration(2.7).
- Encourage patients to void immediately prior to imaging the lumbar spine and bony pelvis(2.7).
DOSAGE FORMS AND STRENGTHS
Multiple-dose vial containing 370 - 7,400 MBq/mL (10 - 200 mCi/mL) of no-carrier-added sodium fluoride F18 at the end of synthesis (EOS) reference time in aqueous 0.9% sodium chloride solution(3). Sodium Fluoride F 18 Injection is a clear, colorless, sterile, pyrogen-free and preservative-free solution for intravenous administration.
CONTRAINDICATIONS
None(4).
WARNINGS AND PRECAUTIONS
- Allergic Reactions: As with any injectable drug product, allergic reactions and anaphylaxis may occur. Emergency resuscitation equipment and personnel should be immediately available.(5.1).
- Cancer Risk: Sodium Fluoride F 18 Injection may increase the risk of cancer. Use the smallest dose necessary for imaging and ensure safe handling to protect the patient and health care worker(5.2).
(5).
ADVERSE REACTIONS
No adverse reactions have been reported for Sodium Fluoride F 18 Injection based on a review of the published literature, publicly available reference sources, and adverse drug reaction reporting systems(6).
To report SUSPECTED ADVERSE REACTIONS, contact SOFIE Co. at 1-800-753-5368 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
- Pregnancy: No human or animal data. Any radiopharmaceutical, including Sodium Fluoride F18 injection, may cause fetal harm. Use only if clearly needed (8.1)
- Nursing: A decision should be made whether to interrupt nursing after Sodium Fluoride F 18 Injection administration or not to administer Sodium Fluoride F 18 Injection taking into consideration the importance of the drug to the mother.(8.3).
- Pediatrics: Children are more sensitive to radiation and may be at higher risk of cancer from Sodium Fluoride F18 injection (8.4).
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 5/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Radiation Safety - Drug Handling
2.2 Radiation Safety - Patient Preparation
2.3 Drug Preparation and Administration
2.4 Recommended Dose for Adults
2.5 Recommended Dose for Pediatric Patients
2.6 Radiation Dosimetry
2.7 Imaging Guidelines
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Allergic Reactions
5.2 Radiation Risks
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
11 DESCRIPTION
11.1 Chemical Characteristics
11.2 Physical Characteristics
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Metastatic Bone Disease
14.2 Other Bone Disorders
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
Storage
Handling
17 PATIENT COUNSELING INFORMATION
17.1 Pre-study Hydration
17.2 Post-study Voiding
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Radiation Safety - Drug Handling
- Wear waterproof gloves and effective shielding when handling Sodium Fluoride F 18 Injection. Use appropriate safety measures, including shielding, consistent with proper patient management to avoid unnecessary radiation exposure to the patient, occupational workers, clinical personnel, and other persons.
- Radiopharmaceuticals should be used by or under the control of physicians who are qualified by specific training and experience in the safe use and handling of radionuclides, and whose experience and training have been approved by the appropriate governmental agency authorized to license the use of radionuclides.
- Use aseptic technique to maintain sterility during all operations involved in the manipulation and administration of Sodium Fluoride F 18 Injection.
- The dose of Sodium Fluoride F 18 Injection should be minimized consistent with the objectives of the procedure, and the nature of the radiation detection devices employed.
- The final dose for the patient should be calculated using proper decay factors from the time of End of Synthesis (EOS), and measured by a suitable radioactivity calibration system before administration [see Description(11.2)].
2.2 Radiation Safety - Patient Preparation
- To minimize the radiation-absorbed dose to the bladder, encourage adequate hydration. Encourage the patient to ingest at least 500 mL of fluid immediately prior and subsequent to the administration of Sodium Fluoride F 18 Injection.
- Encourage the patient to void one-half hour after administration of Sodium Fluoride F 18 Injection and as frequently thereafter as possible for the next 12 hours.
2.3 Drug Preparation and Administration
- Calculate the necessary volume to administer based on calibration time and dose.
- Inspect Sodium Fluoride F 18 Injection visually for particulate matter and discoloration before administration, whenever solution and container permit.
- Do not administer Sodium Fluoride F 18 Injection containing particulate matter or discoloration; dispose of these unacceptable or unused preparations in a safe manner, in compliance with applicable regulations.
- Aseptically withdraw Sodium Fluoride F 18 Injection from its container.
2.5 Recommended Dose for Pediatric Patients
In reported clinical experience in approximately 100 children, weight based doses (2.1 MBq/kg) ranging from 19 MBq– 148 MBq (0.5 mCi- 4 mCi) were used.
2.6 Radiation Dosimetry
The age/weight- based estimated absorbed radiation doses (mGy/MBq) from intravenous injection of Sodium Fluoride F 18 Injection are shown in Table 1. These estimates were calculated based on human data and using the data published by the Nuclear Regulatory Commission [1] and the International Commission on Radiological Protection for Sodium Fluoride Injection [2]. The bone, bone marrow and urinary bladder are considered target and critical organs.
Table 1: Estimated Absorbed Radiation Doses after Intravenous Administration of Sodium Fluoride F 18 Injection Estimated Radiation Dose mGy/MBq Organ Adult
70 kg*15 year
56.8 kg†10 year
33.2 kg†5 year
19.8 kg†1 year
9.7 kg†- * Data from Nuclear Regulatory Commission Report, Radiation Dose Estimates for Radiopharmaceuticals, NUREG/CR-6345, page 10, 1996.
- † Data from ICRP publication 53, Radiation Dose to Patients from Radiopharmaceuticals (), Ann ICRP, Volume 18, , pages15 and 74, 1987
Adrenals 0.0062 0.012 0.018 0.028 0.052 Brain 0.0056 N/A N/A N/A N/A Bone surfaces 0.060 0.050 0.079 0.13 0.30 Breasts 0.0028 0.0061 0.0097 0.015 0.030 GI Gallbladder wall 0.0044 N/A N/A N/A N/A Stomach wall 0.0038 0.008 0.013 0.019 0.036 Small intestine 0.0066 0.012 0.018 0.028 0.052 Upper large intestine wall 0.0058 0.010 0.016 0.026 0.046 Lower large intestine wall 0.012 0.016 0.025 0.037 0.063 Heart wall 0.0039 N/A N/A N/A N/A Kidneys 0.019 0.025 0.036 0.053 0.097 Liver 0.0040 0.0084 0.013 0.021 0.039 Lungs 0.0041 0.0084 0.013 0.020 0.039 Muscle 0.0060 N/A N/A N/A N/A Ovaries 0.011 0.016 0.023 0.036 0.063 Pancreas 0.0048 0.0096 0.015 0.023 0.044 Red marrow 0.028 0.053 0.088 0.18 0.38 Skin 0.0040 N/A N/A N/A N/A Spleen 0.0042 0.0088 0.014 0.021 0.041 Testes 0.0078 0.013 0.021 0.033 0.062 Thymus 0.0035 N/A N/A N/A N/A Thyroid 0.0044 0.0084 0.013 0.020 0.036 Urinary bladder wall 0.25 0.27 0.4 0.61 1.1 Uterus 0.019 0.023 0.037 0.057 0.099 Other tissue N/A 0.010 0.015 0.024 0.044 Effective Dose
Equivalent mSv/MBq0.027 0.034 0.052 0.086 0.17 -
3 DOSAGE FORMS AND STRENGTHS
Multiple-dose vial containing 370 - 7,400 MBq/mL (10 - 200 mCi/mL) at EOS reference time of no-carrier-added sodium fluoride F18 in aqueous 0.9% sodium chloride solution. Sodium Fluoride F 18 Injection is a clear, colorless, sterile, pyrogen-free and preservative-free solution for intravenous administration.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Allergic Reactions
As with any injectable drug product, allergic reactions and anaphylaxis may occur. Emergency resuscitation equipment and personnel should be immediately available.
5.2 Radiation Risks
Sodium Fluoride F 18 Injection may increase the risk of cancer. Carcinogenic and mutagenic studies with Sodium Fluoride F18 injection have not been performed. Use the smallest dose necessary for imaging and ensure safe handling to protect the patient and health care worker [see Dosage and Administration(2.1)].
- 6 ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
Any radiopharmaceutical including Sodium Fluoride F18 Injection has a potential to cause fetal harm. The likelihood of fetal harm depends on the stage of fetal development, and the radionuclide dose. Animal reproduction and developmental toxicity studies have not been conducted with Sodium Fluoride F 18 Injection. Prior to the administration of Sodium Fluoride F 18 Injection to women of childbearing potential, assess for presence of pregnancy. Sodium Fluoride F 18 Injection should be given to a pregnant woman only if clearly needed.8.3 Nursing Mothers
It is not known whether Sodium Fluoride F 18 Injection is excreted into human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants, a decision should be made whether to interrupt nursing after administration of Sodium Fluoride F 18 Injection or not to administer Sodium Fluoride F 18 Injection, taking into account the importance of the drug to the mother. The body of scientific information related to radioactivity decay, drug tissue distribution and drug elimination shows that less than 0.01% of the radioactivity administered remains in the body after 24 hours (10 half-lives). To minimize the risks to a nursing infant, interrupt nursing for at least 24 hours.
8.4 Pediatric Use
In reported clinical experience in approximately 100 children, weight based doses (2.1 MBq/kg) ranging from 19 MBq – 148 MBq (0.5 mCi - 4 mCi) were used. Sodium Fluoride F18 was shown to localize to areas of bone turnover including rapidly growing epiphyses in developing long bones. Children are more sensitive to radiation and may be at higher risk of cancer from Sodium Fluoride F18 injection.
-
11 DESCRIPTION
11.1 Chemical Characteristics
Sodium Fluoride F 18 Injection is a positron emitting radiopharmaceutical, containing no-carrier-added, radioactive fluoride F18 that is used for diagnostic purposes in conjunction with PET imaging. It is administered by intravenous injection. The active ingredient, sodium fluoride F18, has the molecular formula Na[18F] with a molecular weight of 40.99, and has the following chemical structure:
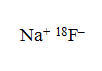
Sodium Fluoride F 18 Injection is provided as a ready-to-use, isotonic, sterile, pyrogen-free, preservative-free, clear and colorless solution. Each mL of the solution contains between 370 MBq and 7,400 MBq (10 mCi to 200 mCi) sodium fluoride F18, at the EOS reference time, in 0.9% aqueous sodium chloride. The pH of the solution is between 4.5 and 8. The solution is presented in XX mL multiple- dose glass vials with variable total volume and total radioactivity in each vial.
11.2 Physical Characteristics
Fluoride F18 decays by positron (β+) emission and has a half-life of 109.7 minutes. Ninety-seven percent of the decay results in emission of a positron with a maximum energy of 633 keV and 3% of the decay results in electron capture with subsequent emission of characteristic X-rays of oxygen. The principal photons useful for diagnostic imaging are the 511 keV gamma photons, resulting from the interaction of the emitted positron with an electron (Table 2). Fluorine F18 atom decays to stable 18O-oxygen.
Table 2: Principal Emission Data for Fluoride F18 Radiation/Emission % per Disintegration Mean Energy - * Produced by positron annihilation
[3] Kocher, D.C. Radioactive Decay Data Tables DOE/TIC-11026, 69, 1981.Positron (β+) 96.73 249.8 keV Gamma (±)* 193.46 511.0 keV The specific gamma ray constant for fluoride F18 is 5.7 R/hr/mCi (1.35 x 10 -6 Gy/hr/kBq) at 1 cm. The half-value layer (HVL) for the 511 keV photons is 4.1 mm lead (Pb). A range of values for the attenuation of radiation results from the interposition of various thickness of Pb. The range of attenuation coefficients for this radionuclide is shown in Table 3. For example, the interposition of an 8.3 mm thickness of Pb with a coefficient of attenuation of 0.25 will decrease the external radiation by 75%.
Table 3:Radiation Attenuation of 511 keV Photons by Lead (Pb) Shielding Shield Thickness (Pb) mm Coefficient of Attenuation 0 0.00 4 0.50 8 0.25 13 0.10 26 0.01 39 0.001 52 0.0001 Table 4 lists the fraction of radioactivity remaining at selected time intervals from the calibration time. This information may be used to correct for physical decay of the radionuclide.
- * Produced by positron annihilation
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Fluoride F18 ion normally accumulates in the skeleton in an even fashion, with greater deposition in the axial skeleton (e.g. vertebrae and pelvis) than in the appendicular skeleton and greater deposition in the bones around joints than in the shafts of long bones.
12.2 Pharmacodynamics
Increased fluoride F18 ion deposition in bone can occur in areas of increased osteogenic activity during growth, infection, malignancy (primary or metastatic) following trauma, or inflammation of bone.
12.3 Pharmacokinetics
After intravenous administration, fluoride F18 ion is rapidly cleared from the plasma in a biexponential manner. The first phase has a half-life of 0.4 h, and the second phase has a half-life of 2.6 h. Essentially all the fluoride F18 that is delivered to bone by the blood is retained in the bone. One hour after administration of fluoride F18 only about 10% of the injected dose remains in the blood. Fluoride F18 diffuses through capillaries into bone extracellular fluid space, where it becomes bound by chemisorption at the surface of bone crystals, preferentially at sites of newly mineralizing bone.
Deposition of fluoride F18 in bone appears to be primarily a function of blood flow to the bone and the efficiency of the bone in extracting the fluoride F18. Fluoride F18 does not appear to be bound to serum proteins.
In patients with normal renal function, 20% or more of the fluorine ion is cleared from the body in the urine within the first 2 hours after intravenous administration.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
14.1 Metastatic Bone Disease
The doses used in reported studies ranged from 2.7 mCi to 20 mCi (100 MBq to 740 MBq), with an average median dose of 10 mCi (370 MBq) and an average mean dose of 9.2 mCi (340 MBq). In PET imaging of bone metastases with Sodium Fluoride F 18 Injection, focally increased tracer uptake is seen in both osteolytic and osteoblastic bone lesions. Negative PET imaging results with Sodium Fluoride F 18 Injection do not preclude the diagnosis of bone metastases. Also, as benign bone lesions are also detected by Sodium Fluoride F 18 Injection, positive PET imaging results cannot replace biopsy to confirm a diagnosis of cancer.
-
15 REFERENCES
- Stabin, M.G., Stubbs, J.B. and Toohey R.E.,Radiation Dose Estimates for Radiopharmaceuticals,U.S. Nuclear Regulatory Commission report NUREG/CR-6345, page 10, 1996.
- Radiation Dose to Patients from Radiopharmaceuticals,ICRP publication 53, Ann ICRP, 18 pages 15 and 74, 1987.
- Kocher, D.C., "Radioactive Decay Data Tables: A Handbook of decay data for application to radiation dosimetry and radiological assessments" DOE/TIC-11026, page 69, 1981.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Sodium Fluoride F 18 Injection is supplied in a multiple-dose Type I glass vial with elastomeric stopper and aluminum crimp seal containing between 370 and 7,400 MBq/mL (10 - 200 mCi/mL) of no-carrier-added sodium fluoride F18, at the EOS reference time, in aqueous 0.9% sodium chloride solution. The total volume and total radioactivity per vial are variable. Each vial is enclosed in a shielding container of appropriate thickness.
The product is available in a 30 mL vial configuration with a variable fill volume. The NDC number is:
49609-102-01 (30 mL) -
17 PATIENT COUNSELING INFORMATION
17.1 Pre-study Hydration
Encourage patients to drink at least 500 mL of water prior to drug administration.
17.2 Post-study Voiding
To help protect themselves and others in their environment, patients should take the following precautions for 12 hours after injection: whenever possible, use a toilet and flush several times after each use; wash hands thoroughly after each voiding or fecal elimination. If blood, urine or feces soil clothing, wash the clothing separately.
- SPL UNCLASSIFIED SECTION
-
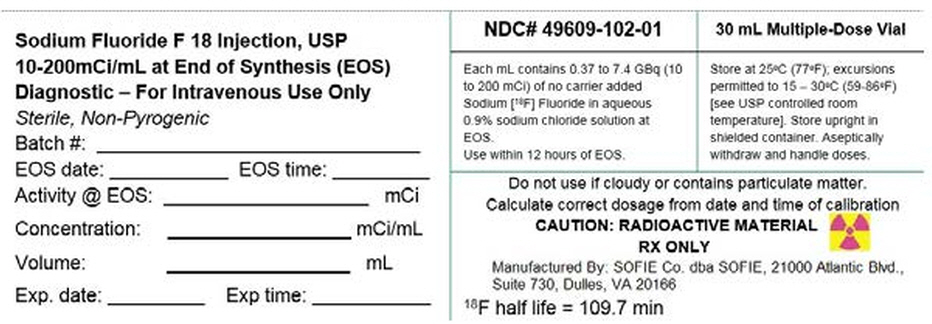
PRINCIPAL DISPLAY PANEL - 30 mL Vial Label
Sodium Fluoride F 18 Injection, USP
10-200mCi/mL at End of Synthesis (EOS)
Diagnostic – For Intravenous Use Only
Sterile, Non-Pyrogenic
Batch #: __________________________
EOS date: ________ EOS time: _______
Activity @ EOS: _______________ mCi
Concentration: ____________ mCi/mL
Volume: _________________ mL
Exp. date: _______ Exp time: ________
NDC# 49609-102-01
Each mL contains 0.37 to 7.4 GBq (10
to 200 mCi) of no carrier added
Sodium [18F] Fluoride in aqueous
0.9% sodium chloride solution at
EOS.
Use within 12 hours of EOS.30 mL Multiple-Dose Vial
Store at 25°C (77°F); excursions
permitted to 15 – 30°C (59-86°F)
[see USP controlled room
temperature]. Store upright in
shielded container. Aseptically
withdraw and handle doses.Do not use if cloudy or contains particulate matter.
Calculate correct dosage from date and time of calibrationCAUTION: RADIOACTIVE MATERIAL
RX ONLY
Manufactured By: SOFIE Co. dba SOFIE, 21000 Atlantic Blvd.,
Suite 730, Dulles, VA 2016618F half life = 109.7 min
-
INGREDIENTS AND APPEARANCE
SODIUM FLUORIDE F 18
sodium fluoride f-18 injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 49609-102 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE F-18 (UNII: 9L75099X6R) (FLUORIDE ION F-18 - UNII:4M4WE5N2GE) FLUORIDE ION F-18 200 mCi in 1 mL Inactive Ingredients Ingredient Name Strength Sodium Chloride (UNII: 451W47IQ8X) 9 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49609-102-01 30 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product 12/08/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA203592 12/08/2011 Labeler - SOFIE Co. (957704737) Establishment Name Address ID/FEI Business Operations N-Molecular, Inc. dba SOFIE 079932600 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(49609-102) Establishment Name Address ID/FEI Business Operations SOFIE Co. dba SOFIE 025600556 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(49609-102) Establishment Name Address ID/FEI Business Operations SOFIE Co. dba SOFIE 832599976 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(49609-102) Establishment Name Address ID/FEI Business Operations N-Molecular, Inc. dba SOFIE 079932640 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(49609-102) Establishment Name Address ID/FEI Business Operations SOFIE Co. dba SOFIE 829109441 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(49609-102) Establishment Name Address ID/FEI Business Operations SOFIE Co. dba SOFIE 078623800 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(49609-102) Establishment Name Address ID/FEI Business Operations SOFIE Co. dba SOFIE 032324142 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(49609-102) Establishment Name Address ID/FEI Business Operations SOFIE Co. dba SOFIE 006320902 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(49609-102) Establishment Name Address ID/FEI Business Operations SOFIE Co. dba SOFIE 008017970 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(49609-102) Establishment Name Address ID/FEI Business Operations SOFIE Co. dba SOFIE 079854636 POSITRON EMISSION TOMOGRAPHY DRUG PRODUCTION(49609-102)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.