ELLA- ulipristal acetate tablet
Ella by
Drug Labeling and Warnings
Ella by is a Prescription medication manufactured, distributed, or labeled by HRA Pharma America, Inc., Laboratoire HRA Pharma, Delpharm Lille S.A.S., CENEXI, Laboratorios Leon Farma S.A.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ella safely and effectively. See full prescribing information for ella.
ELLA (ulipristal acetate) tablet, for oral use
Initial U.S. Approval: 2010RECENT MAJOR CHANGES
INDICATIONS AND USAGE
ella is a progesterone agonist/antagonist emergency contraceptive indicated for prevention of pregnancy following unprotected intercourse or a known or suspected contraceptive failure. ella is not intended for routine use as a contraceptive. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
-
30 mg tablet (3)
CONTRAINDICATIONS
- Known or suspected pregnancy (4)
WARNINGS AND PRECAUTIONS
- ella is not indicated for termination of an existing pregnancy. (5.1)
- Subsequent acts of intercourse should be protected by a reliable barrier method until next menstrual period. If a woman wishes to use hormonal contraception, she should do so no sooner than 5 days after intake of ella. (5.5)
- Ectopic pregnancy: Women who become pregnant or complain of lower abdominal pain after taking ella should be evaluated for ectopic pregnancy. (5.2)
- Effect on menstrual cycle: ella may alter the next expected menses. If menses is delayed beyond 1 week, pregnancy should be ruled out. (5.6)
- ella does not protect against STI/HIV. (5.7)
ADVERSE REACTIONS
The most common adverse reactions (≥ 5%) in the clinical trials were headache (18%), abdominal pain (12%), nausea (12%), dysmenorrhea (9%), fatigue (6%) and dizziness (5%). (6)
To report SUSPECTED ADVERSE REACTIONS, contact HRA Pharma America Inc., at 844-994-0329 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS
- Drugs or herbal products that induce CYP3A4 decrease the effectiveness of ella. (7)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 5/2020
-
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Existing Pregnancy
5.2 Ectopic Pregnancy
5.3 Repeated Use
5.4 CYP3A4 Inducers
5.5 Fertility Following Use
5.6 Effect on Menstrual Cycle
5.7 Sexually Transmitted Infections/HIV
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Changes in Emergency Contraceptive Effectiveness Associated with Co-Administration of Other Products
7.2 Increase in Plasma Concentrations of ella Associated with Co-Administered Drugs
7.3 Effects of ella on Co-Administered Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Race
8.7 Hepatic Impairment
8.8 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Open-Label Study
14.2 Single-Blind Comparative Study
14.3 Pooled Analysis
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
Instruct patients to take one tablet orally as soon as possible within 120 hours (5 days) after unprotected intercourse or a known or suspected contraceptive failure.
The tablet can be taken with or without food.
If vomiting occurs within 3 hours of ella intake, consideration should be given to repeating the dose.
ella can be taken at any time during the menstrual cycle.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
ella is contraindicated for use in the case of known or suspected pregnancy. [See Use in Specific Populations (8.1).]
-
5 WARNINGS AND PRECAUTIONS
5.2 Ectopic Pregnancy
A history of ectopic pregnancy is not a contraindication to use of this emergency contraceptive method. Healthcare providers, however, should consider the possibility of ectopic pregnancy in women who become pregnant or complain of lower abdominal pain after taking ella. A follow-up physical or pelvic examination is recommended if there is any doubt concerning the general health or pregnancy status of any woman after taking ella.
5.3 Repeated Use
ella is for occasional use as an emergency contraceptive. It should not replace a regular method of contraception. Repeated use of ella within the same menstrual cycle is not recommended, as safety and efficacy of repeat use within the same cycle has not been evaluated.
5.4 CYP3A4 Inducers
A CYP3A4 inducer, rifampin, decreases the plasma concentration of ella significantly. ella should not be administered with CYP3A4 inducers [see Drug interactions (7.1) and Clinical Pharmacology (12.3)].
5.5 Fertility Following Use
A rapid return of fertility is likely following treatment with ella for emergency contraception.
After use of ella, a reliable barrier method of contraception should be used with subsequent acts of intercourse that occur in that same menstrual cycle.
Because ella and the progestin component of hormonal contraceptives both bind to the progesterone receptor, using them together could reduce their contraceptive effect. After using ella, if a woman wishes to use hormonal contraception, she should do so no sooner than 5 days after the intake of ella, and she should use a reliable barrier method until the next menstrual period [see Drug Interactions (7.1 and 7.3)and Clinical Pharmacology (12.2)].
5.6 Effect on Menstrual Cycle
After ella intake, menses sometimes occur earlier or later than expected by a few days. In clinical trials, cycle length was increased by a mean of 2.5 days but returned to normal in the subsequent cycle. Seven percent of subjects reported menses occurring more than 7 days earlier than expected, and 19% reported a delay of more than 7 days. If there is a delay in the onset of expected menses beyond 1 week, rule out pregnancy.
Nine percent of women studied reported intermenstrual bleeding after use of ella.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
ella was studied in an open-label multicenter trial (Open-Label Study) and in a comparative, randomized, single-blind, multicenter trial (Single-Blind Comparative Study). In these studies, a total of 2,637 (1,533 + 1,104) women in the 30 mg ulipristal acetate groups were included in the safety analysis. The mean age of women who received ulipristal acetate was 24.5 years and the mean body mass index (BMI) was 25.3. The racial demographics of those enrolled were 67% Caucasian, 20% Black or African American, 2% Asian, and 12% other.
The most common adverse reactions (≥ 10%) in the clinical trials for women receiving ella were headache (18% overall) and nausea (12% overall) and abdominal and upper abdominal pain (12% overall). Table 1 lists those adverse reactions that were reported in ≥ 5% of subjects in the clinical studies (14).
Table 1: Adverse Reactions in ≥ 5% of Women (%) Receiving a Single Dose of ella (30 mg Ulipristal Acetate) Most Common
Adverse ReactionsOpen-Label
StudySingle-Blind
Comparative
StudyN = 1,533 N = 1,104 Headache 18 19 Nausea 12 13 Abdominal and upper abdominal pain 15 8 Dysmenorrhea 7 13 Fatigue 6 6 Dizziness 5 5 6.2 Postmarketing Experience
Adolescents: the safety profile observed in adolescents aged 17 and younger in studies and post-marketing is similar to the safety profile in adults [see Pediatric Use (8.4)].
The following adverse reactions have been identified during post-approval use of ella:
Skin and Subcutaneous Tissue Disorders: Acne
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
-
7 DRUG INTERACTIONS
Several in vivo drug interaction studies have shown that ella is predominantly metabolized by CYP3A4.
7.1 Changes in Emergency Contraceptive Effectiveness Associated with Co-Administration of Other Products
Drugs or herbal products that induce CYP3A4 decrease the plasma concentrations of ella, and may decrease its effectiveness [see Warnings and Precautions (5.4) and Clinical Pharmacology (12.3)]. Avoid co-administration of ella and drugs or herbal products such as:
- barbiturates
- bosentan
- carbamazepine
- felbamate
- griseofulvin
- oxcarbazepine
- phenytoin
- rifampin
- St. John's Wort
- topiramate
Hormonal contraceptives: Progestin-containing contraceptives may impair the ability of ella to delay ovulation [see Warnings and Precautions (5.5) and Clinical Pharmacology (12.2)]. Avoid co-administration of ella and hormonal contraceptives. If a woman wishes to start or resume hormonal contraception after the intake of ella, she should do so no sooner than 5 days afterwards, and she should use a reliable barrier method until the next menstrual period.
7.2 Increase in Plasma Concentrations of ella Associated with Co-Administered Drugs
CYP3A4 inhibitors such as itraconazole or ketoconazole increase plasma concentrations of ella [see Pharmacokinetics (12.3)].
7.3 Effects of ella on Co-Administered Drugs
Hormonal contraceptives: ella may impact the effect of the progestin component of hormonal contraceptives. Therefore, if a woman wishes to use hormonal contraception after using ella, she should use a reliable barrier method for subsequent acts of intercourse until her next menstrual period [see Warnings and Precautions (5.5) and Clinical Pharmacology(12.2)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
ella is contraindicated for use during an existing or suspected pregnancy. No signal of concern regarding pregnancy complications was found in postmarketing studies [seeData]. Isolated cases of major malformations in ella-exposed pregnancies were identified; however, the data are not sufficient to determine a risk for birth defects with inadvertent use of ella during pregnancy. Miscarriage was reported in 14% of the known pregnancy outcomes; a rate that is similar to the U.S background rate for miscarriage. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
In animal reproduction studies, no malformations were observed during repeated administration of ulipristal acetate to pregnant rats, rabbits and monkeys at daily drug exposures ⅓, ½, and 3 times respectively, the human exposure at a dose of 30 mg [see Data] .
Data
Human Data
ella pregnancy exposure data was collected in the U.S. and Europe from 1999 to 2015 and analyzed post-marketing using data from interventional clinical trials, observational studies and pharmacovigilance reports. Known pregnancy outcomes were available for 462/784 pregnancies in which women received ella at doses of 30 mg or greater during the conception cycle or during pregnancy. Data of pregnancies with known outcome were analyzed prospectively for 272 cases and retrospectively for 190 cases. Pregnancy outcomes included 302 elective abortions (2 for fetal anomalies including 1 with trisomy 21), 63 spontaneous abortions, and 13 ectopic pregnancies. No maternal or fetal deaths were reported. 84 pregnancies continued until birth, with congenital anomalies reported in 5 infants, including 4 major malformations (2/4 with genetic syndromes). Although these data do not allow estimation of the prevalence rate of congenital anomalies associated with inadvertent use of ella in pregnancy or determination of a causal relationship between reported anomalies and ella, they show that ella-exposed pregnancies were not associated with a pattern of increased risk of adverse outcomes.
Animal Data
Ulipristal acetate was administered repeatedly to pregnant rats and rabbits during the period of organogenesis. Embryofetal loss was noted in all pregnant rats and in half of the pregnant rabbits following 12 and 13 days of dosing, at daily drug exposures 1/3 and 1/2 the human exposure, respectively, based on body surface area (mg/m2). There were no malformations of the surviving fetuses in these studies. Adverse effects were not observed in the offspring of pregnant rats administered ulipristal acetate during the period of organogenesis through lactation at drug exposures 1/24 the human exposure based on AUC. Administration of ulipristal acetate to pregnant monkeys for 4 days during the first trimester caused pregnancy termination in 2/5 animals at daily drug exposures 3 times the human exposure based on body surface area.
8.2 Lactation
Risk Summary
Ulipristal acetate and its active metabolite, monodemethyl-ulipristal acetate, are present in human milk in small
amounts (see Data). Based on the levels of drug and active metabolite measured in breastmilk, a fully breastfed child would receive a weight-adjusted dosage of approximately 0.8% of ulipristal acetate and monodemethyl-ulipristal acetate on Day 1 of drug administration and an approximate total of 1% of the maternal dose over a 5-day period after drug administration. There is no information on the effects on the breastfed child or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for ella and any potential adverse effects on the breastfed child from ella or from the underlying maternal condition.Data
The breast milk of 12 lactating women following administration of ella was collected in 24-hour increments to measure the concentrations of ulipristal acetate and the active metabolite monodemethyl-ulipristal acetate in breast milk. The mean daily concentrations of ulipristal acetate in breast milk were 22.7 ng/mL [0-24 hours], 2.96 ng/mL [24-48 hours], 1.56 ng/mL [48-72 hours], 1.04 ng/mL [72-96 hours], and 0.69 ng/mL [96-120 hours]. The mean daily concentrations of monodemethyl-ulipristal acetate in breast milk were 4.49 ng/mL [0-24 hours], 0.62 ng/mL [24-48 hours], 0.28 ng/mL [48-72 hours], 0.17 ng/mL [72-96 hours], and 0.10 ng/mL [96-120 hours]. Using these data, a fully breastfed infant would receive approximately 4.1 mcg/kg of ulipristal acetate and monodemethyl-ulipristal acetate on Day 1 following drug administration and approximately 5.2 mcg/kg over a five day period following drug administration.
8.3 Females and Males of Reproductive Potential
Contraception
ella and progestin-containing contraceptives' may interact and decrease the effectiveness of both products. Advise females to use a a reliable barrier method for subsequent acts of intercourse until her next menstrual period and to wait at least 5 days after taking ella to resume oral contraceptives [see Warnings and Precautions (5.5), Drug Interactions (7), and Clinical Pharmacology (2.2, 12.3)].
8.4 Pediatric Use
Safety and efficacy of ella have been established in women of reproductive age. The clinical trials of ella enrolled 41 females under age 18, and a post-marketing observational study evaluating effectiveness and safety of ella in adolescents enrolled 279 females under age 18, including 76 under age 16 years. In these studies, the safety and efficacy profile observed in adolescents aged 17 and younger was similar to that in adults. Use of ella before menarche is not indicated.
8.6 Race
While no formal studies have evaluated the effect of race, a cross-study comparison of two pharmacokinetic studies indicated that exposure in South Asians may exceed that in Caucasians and African Americans. However, no difference in efficacy and safety was observed for women of different races in clinical studies.
- 10 OVERDOSAGE
-
11 DESCRIPTION
The ella (ulipristal acetate) tablet for oral use contains 30 mg of a single active steroid ingredient, ulipristal acetate [17α-acetoxy-11β-(4-N,N-dimethylaminophenyl)-19-norpregna-4,9-diene-3,20-dione], a synthetic progesterone agonist/antagonist. The inactive ingredients are lactose monohydrate, povidone K-30, croscarmellose sodium and magnesium stearate.
Ulipristal acetate is a white to yellow crystalline powder which has a molecular weight of 475.6. The structural formula is:
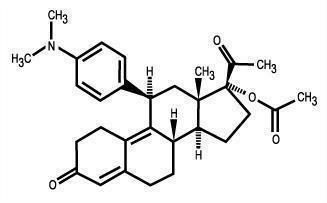
C30H37NO4
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
When taken immediately before ovulation is to occur, ella postpones follicular rupture. The likely primary mechanism of action of ulipristal acetate for emergency contraception is therefore inhibition or delay of ovulation; however, alterations to the endometrium that may affect implantation may also contribute to efficacy.
12.2 Pharmacodynamics
Ulipristal acetate is a selective progesterone receptor modulator with antagonistic and partial agonistic effects (a progesterone agonist/antagonist) at the progesterone receptor. It binds the human progesterone receptor and prevents progesterone from occupying its receptor.
The pharmacodynamics of ulipristal acetate depends on the timing of administration in the menstrual cycle. Administration in the mid-follicular phase causes inhibition of folliculogenesis and reduction of estradiol concentration.
Pharmacodynamic data showed that administration of ella to 34 women in the late follicular phase postponed follicular rupture for at least 5 days in all (100%) of 8 subjects who took ella before the luteinizing hormone (LH) surge and 11 (79%) of 14 subjects who took ella immediately before ovulation (when LH has already started to rise). However, treatment was not effective in postponing follicular rupture when administered on the day of LH peak.
Dosing in the early luteal phase does not significantly delay endometrial maturation but decreases endometrial thickness by 0.6 ± 2.2 mm (mean ± SD).
Hormonal Contraceptives after ella intake:
When a combined oral contraceptive pill (COC) containing ethinyl estradiol 30 µg + levonorgestrel 150 µg was started the day after ella intake during the follicular phase, ella did not interfere with the COC's ability to suppress ovarian activity, as assessed by measurement of follicle size via transvaginal ultrasound, combined with serum progesterone and estradiol levels: ovarian activity was suppressed in 61.5% (24/39) of subjects receiving ella plus COC and 62.2% (23/37) of subjects receiving a placebo plus the COC. The incidence of ovulation was similar between the group who received ella plus the COC [33.3% (13/39)] and the group who received a placebo plus the COC [32.4% (12/37)]. [see Warnings and Precautions (5.5) and Drug Interactions (7.3)].
The initiation of a desogestrel 75 µg "progestin-only pill" the day after ella intake during the follicular phase was associated with a higher incidence of ovulation in the six days following ella intake compared to an ella-only treatment group, and a relatively slower onset (3 to 4 days) of thickened cervical mucus compared to a group given desogestrel without prior ella intake (2 days), suggesting an effect of prior use of ella on the ability of desogestrel to inhibit mucus permeability. [See Warnings and Precautions (5.5) and Drug Interactions (7.1; 7.3)].
12.3 Pharmacokinetics
Absorption
Following a single dose administration of ella in 20 women under fasting conditions, maximum plasma concentrations of ulipristal acetate and the active metabolite, monodemethyl-ulipristal acetate, were 176 and 69 ng/ml and were reached at 0.9 and 1 hour, respectively.
Figure 1: Mean (± SD) Plasma Concentration-time Profile of Ulipristal Acetate and Monodemethyl-ulipristal Acetate Following Single Dose Administration of 30 mg Ulipristal Acetate
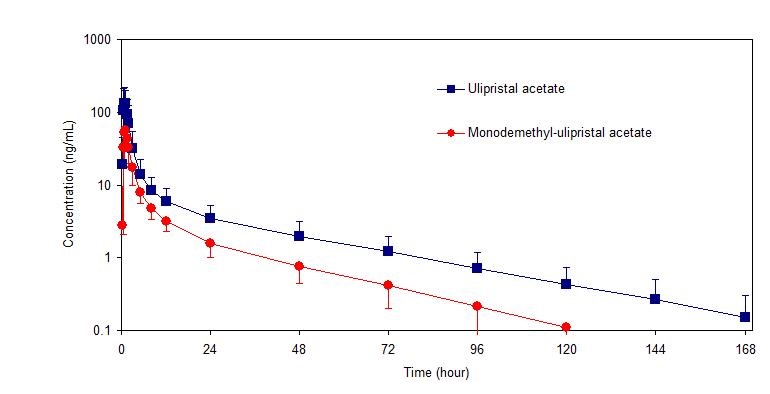
Table 2: Pharmacokinetic Parameter Values Following Administration of ella (ulipristal acetate) Tablet 30 mg to 20 Healthy Female Volunteers under Fasting Conditions Mean (± SD) Cmax
(ng/ml)AUC0-t
(nghr/ml)AUC0-∞
(nghr/ml)tmax
(hr)*t1/2
(hr)Ulipristal acetate 176
(89)548
(259)556
(260)0.9
(0.5-2.0)32
(6.3)Monodemethyl-
ulipristal acetate69
(26)240
(59)246
(59)0.9
(0.8-2.0)27
(6.9)Cmax = maximum concentration
AUC0-t = area under the drug concentration curve from time 0 to time of last determinable concentration
AUC0-∞ = area under the drug concentration curve from time 0 to infinity
tmax = time to maximum concentration
t1/2 = elimination half-life
* Median (range)Effect of food: Administration of ella together with a high-fat breakfast resulted in approximately 40 - 45% lower mean Cmax, a delayed tmax (from a median of 0.75 hours to 3 hours) and 20 - 25% higher mean AUC0-∞ of ulipristal acetate and monodemethyl-ulipristal acetate compared with administration in the fasting state. These differences are not expected to impair the efficacy or safety of ella to a clinically significant extent; therefore, ella can be taken with or without food.
Distribution
Ulipristal acetate is highly bound (> 94%) to plasma proteins, including high density lipoprotein, alpha-l-acid glycoprotein, and albumin.
Metabolism
Ulipristal acetate is metabolized to mono-demethylated and di-demethylated metabolites. In vitro data indicate that this is predominantly mediated by CYP3A4. The mono-demethylated metabolite is pharmacologically active.
Excretion
The terminal half-life of ulipristal acetate in plasma following a single 30 mg dose is estimated to 32.4 ± 6.3 hours.
Drug interactions
CYP3A4 inducers: When a single 30 mg dose of ulipristal acetate was administered following administration of the strong CYP3A4 inducer, rifampin 600 mg once daily for 9 days, Cmax and AUC of ulipristal acetate decreased by 90% and 93% respectively. The Cmax and AUC of monodemethyl-ulipristal acetate decreased by 84% and 90% respectively [see Drug Interactions (7.1)].
CYP3A4 inhibitors: When a single 10 mg dose of ulipristal acetate was administered following administration of the strong CYP3A4 inhibitor, ketoconazole 400 mg once daily for 7 days, Cmax and AUC of ulipristal acetate increased by 2- and 5.9- fold, respectively. While the AUC of monodemethyl-ulipristal acetate increased by 2.4-fold, Cmax of monodemethyl-ulipristal acetate decreased by 47%. There was no in vivo drug-drug interaction study between ulipristal acetate 30 mg and CYP3A4 inhibitors [see Drug Interactions (7.1)].
In vitro studies demonstrated that ella does not induce or inhibit the activity of cytochrome P450 enzymes.
P-glycoprotein (P-gp) transporter: In vitro data indicate that ulipristal may be an inhibitor of P-gp at clinically relevant concentrations. When a single 60 mg dose of fexofenadine, a substrate of P-gp glycoprotein, was administered 1.5 hours after the administration of a single 10 mg dose of ulipristal acetate, there was no increase in Cmax or AUC of fexofenadine.
Breast Cancer Resistance Protein (BCRP) transporter: In vitro data indicate that ulipristal acetate may be an inhibitor of BCRP at the intestinal level.
The effects of ella on P-gp and BCRP transporters are unlikely to have any clinical consequences when considering ella's single dose treatment regimen, although there was no in vivo drug interaction study between ulipristal acetate 30 mg (ella) and substrates of P-pg and BCRP transporters.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity:
Carcinogenicity potential was evaluated in rats and mice.
Sprague Dawley rats were exposed to ulipristal acetate daily for 99-100 weeks at doses of 1, 3, or 10 mg/kg/day, representing exposures up to 31 times higher than exposures at the maximum recommended human dose (MRHD). There were no drug-related neoplasms in male rats. In female rats, potential treatment-related neoplastic findings were limited to adrenal cortical adenomas in the intermediate dose group (3 mg/kg/day). Despite the increase, this incidence of adrenal cortical adenomas in females may not be relevant to clinical use.
Tg.rasH2 transgenic mice were exposed to ulipristal acetate for 26 weeks at doses of 5, 45, or 130 mg/kg/day, representing exposures 100 times higher than exposures at the MRHD. There was no drug-related increase in neoplasm incidence in male or female mice.
Genotoxicity: Ulipristal acetate was not genotoxic in the Ames assay, in vitro mammalian assays utilizing mouse lymphoma cells and human peripheral blood lymphocytes , and in an in vivo micronucleus assay in mice.
Impairment of Fertility: Single oral doses of ulipristal acetate prevented ovulation in 50% of rats at 2 times the human exposure based on body surface area (mg/m2). Single doses of ulipristal acetate given on post-coital days 4 or 5 prevented pregnancy in 80-100% of rats and in 50% of rabbits when given on post-coital days 5 or 6 at drug exposures 4 and 12 times the human exposure based on body surface area. Lower doses administered for 4 days to rats and rabbits were also effective at preventing ovulation and pregnancy.
-
14 CLINICAL STUDIES
Two multicenter clinical studies evaluated the efficacy and safety of ella. An open-label study provided the primary data to support the efficacy and safety of ulipristal acetate for emergency contraception when taken 48 to 120 hours after unprotected intercourse. A single-blind comparative study provided the primary data to support the efficacy and safety of ulipristal acetate for emergency contraception when taken 0 to 72 hours after unprotected intercourse and provided supportive data for ulipristal acetate for emergency contraception when taken > 72 to 120 hours after unprotected intercourse. Women in both studies were required to have a negative pregnancy test prior to receiving emergency contraception. The primary efficacy analyses were performed on subjects less than 36 years of age who had a known pregnancy status after taking study medication.
Table 3: Summary of Clinical Trial Results for Women Who Received a Single Dose of ella (30 mg Ulipristal Acetate) Open-Label Study
48 to 120 Hours *Single-Blind Comparative Study
0 to 72 Hours *N = 1,242 N = 844 Expected Pregnancy Rate ** 5.5 5.6 Observed Pregnancy Rate **
(95% confidence interval)2.2
(1.5, 3.2)1.9
(1.1, 3.1)* Time after unprotected intercourse when ella was taken
** Number of pregnancies per 100 women at risk for pregnancy14.1 Open-Label Study
This study was a multicenter open-label trial conducted at 40 family planning clinics in the United States. Healthy women with a mean age of 24 years who requested emergency contraception 48 to 120 hours after unprotected intercourse received a dose of 30 mg ulipristal acetate (ella). The median BMI for the study subjects was 25.3 and ranged from 16.1 to 61.3 kg/m2.
Twenty-seven pregnancies occurred in 1,242 women aged 18 to 35 years evaluated for efficacy. The number of pregnancies expected without emergency contraception was calculated based on the timing of intercourse with regard to each woman's menstrual cycle. ella statistically significantly reduced the pregnancy rate, from an expected rate of 5.5% to an observed rate of 2.2%, when taken 48 to 120 hours after unprotected intercourse.
14.2 Single-Blind Comparative Study
This study was a multicenter, single-blind, randomized comparison of the efficacy and safety of 30 mg ulipristal acetate (ella) to levonorgestrel (another form of emergency contraception). Subjects were enrolled at 35 sites in the U.S., the United Kingdom and Ireland, with the majority (66%) having been enrolled in the U.S. Healthy women with a mean age of 25 years who requested emergency contraception within 120 hours of unprotected intercourse were enrolled and randomly allocated to receive ella or levonorgestrel 1.5 mg. The median BMI for the study subjects was 25.3 and ranged from 14.9 to 70.0 kg/m2.
In the ella group, 16 pregnancies occurred in 844 women aged 16 to 35 years when emergency contraception was taken 0 to 72 hours after unprotected intercourse. The number of pregnancies expected without emergency contraception was calculated based on the timing of intercourse with regard to each woman's menstrual cycle; ella statistically significantly reduced the pregnancy rate, from an expected 5.6% to an observed 1.9%, when taken within 72 hours after unprotected intercourse. There were no pregnancies observed in the women who were administered ella more than 72 hours after unprotected intercourse (10% of women who received ella).
14.3 Pooled Analysis
Data from the two studies were pooled to provide a total efficacy population of women treated with ulipristal acetate up to 120 hours after UPI. Time Trend analysis for the five 24-hour intervals from 0 to 120 hours between unprotected intercourse and treatment was conducted. There were no significant differences in the observed pregnancy rates across the five time intervals.
Subgroup analysis of the pooled data by BMI showed that for women with BMI > 30 kg/m2 (16% of all subjects), the observed pregnancy rate was 3.1% (95% CI: 1.7, 5.7), which was not significantly reduced compared to the expected pregnancy rate of 4.5% in the absence of emergency contraception taken within 120 hours after unprotected intercourse. In the comparative study, a similar effect was seen for the comparator emergency contraception drug, levonorgestrel 1.5 mg. For levonorgestrel, when used by women with BMI > 30 kg/m2, the observed pregnancy rate was 7.4% (95% CI: 3.9, 13.4), compared to the expected pregnancy rate of 4.4% in the absence of emergency contraception taken within 72 hours after unprotected intercourse.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
ella (ulipristal acetate) tablet, 30 mg is supplied in a PVC-PE-PVDC or PVC-PVDC-aluminum blister. The tablet is a white to off-white, round, curved tablet marked with "ella" on both sides.
NDC: 73302-456-01 (1 tablet unit of use package)
Store at 20-25°C (68-77°F).
[See USP controlled room temperature.]Keep the blister in the outer carton in order to protect from light. Keep out of reach of children.
-
17 PATIENT COUNSELING INFORMATION
[See FDA- Approved Patient Labeling]
Information for Patients
- Instruct patients to take ella as soon as possible and not more than 120 hours after unprotected intercourse or a known or suspected contraceptive failure.
- Advise patients that they should not take ella if they know or suspect they are pregnant and that ella is not indicated for termination of an existing pregnancy.
- Advise patients to contact their healthcare provider immediately in case of vomiting within 3 hours of taking the tablet, to discuss whether to take another tablet.
- Advise patients to seek medical attention if they experience severe lower abdominal pain 3 to 5 weeks after taking ella, in order to be evaluated for an ectopic pregnancy.
- Advise patients to contact their healthcare provider and consider the possibility of pregnancy if their period is delayed after taking ella by more than 1 week beyond the date it was expected.
- Advise patients not to use ella as routine contraception, or to use it repeatedly in the same menstrual cycle.
- Advise patients that using ella and hormonal contraceptives together can affect the effectiveness of each. Advise patients to use a reliable barrier method for all subsequent acts of intercourse until the next menstrual period. If a woman wishes to use hormonal contraception, she should do so no sooner than 5 days after intake of ella, and she should use a reliable barrier method until the next menstrual period.
- Advise patients not to use ella if they are taking a CYP3A4 inducer.
- Inform patients that ella does not protect against HIV infection (AIDS) and other sexually transmitted diseases/infections.
-
FDA-Approved Patient Labeling
FDA-Approved Patient Labeling Patient Information
ella ("el-uh")
(ulipristal acetate) tabletRead this Patient Information Leaflet before you take ella. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
What is ella?
ella is a prescription emergency contraceptive that reduces your chance of becoming pregnant if your birth control fails or you have unprotected sex.
ella should not be used as your regular birth control. It is very important that you have a reliable form of birth control that is right for you.
ella will not protect you against HIV infection (AIDS) and other sexually transmitted diseases (STDs).Who should not take ella?
- Do not take ella if you know or suspect you are already pregnant. ella is not for use to end an existing pregnancy.
What should I tell my healthcare provider before taking ella?
See "Who should not take ella?"
Tell your healthcare provider about all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements.Using some other medicines may make ella less effective. These include St. John's Wort, bosentan, griseofulvin, phenytoin, topiramate, oxcarbazepine, carbamazepine, barbitarates, rifampin, and felbamate. Talk to your healthcare provider about whether ella is right for you if you are currently using these medications. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
What should I do about birth control after I take ella?
Using ella with hormonal contraceptives such as birth control pills could reduce the effectiveness of both drugs to prevent pregnancy. After using ella, if you wish to use hormonal contraception, you should do so no sooner than 5 days after the intake of ella. Be sure to use a reliable barrier contraceptive method (such as a condom with spermicide) each time you have sex until your hormonal birth control has taken effect.
If you do not use hormonal contraception, after using ella, you should use a reliable barrier contraceptive method (such as condom with spermicide) each time you have sex.
When is it not appropriate to use ella?
- Do not use ella as a regular birth control method. It does not work as well as most other forms of birth control when they are used consistently and correctly.
- Do not use ella if you are already pregnant.
- Do not use ella more than one time in the same menstrual cycle for different acts of unprotected sex or birth control failure.
How does ella work?
ella is thought to work for emergency contraception primarily by stopping or delaying the release of an egg from the ovary. It is possible that ella may also work by preventing attachment (implantation) to the uterus.
How should I take ella?
- Take ella as soon as possible within 5 days (120 hours) after unprotected sex or if you had a birth control failure.
- ella can be taken with or without food.
- Contact your healthcare provider right away if you vomit within 3 hours of taking ella. Your healthcare provider may prescribe another dose of ella for you.
- ella can be taken at any time during the menstrual cycle.
How effective is ella?
If ella is taken as directed, it will reduce the chance that you will get pregnant. ella is not effective in every case. ella is only to be used for a single episode of unprotected intercourse. Be sure to use a regular birth control method the next time you have sex.
ella and other emergency contraceptives may be less effective in women with a body mass index (BMI) > 30 kg/m2.What if I am already pregnant and use ella?
ella should not be taken if you are already pregnant. Currently there is no information suggesting that ella would harm a developing baby. Contact your healthcare provider if you think you may be pregnant and have taken ella.
ella is not for use to terminate an existing pregnancy.What should I do if my menstrual period is delayed beyond 1 week or I have severe lower stomach (abdominal) pain?
After taking ella, your next menstrual period may begin a few days earlier or later than expected. If your period is more than 7 days later than expected, you may be pregnant. You should get a pregnancy test and follow up with your healthcare provider.
If you have severe lower stomach (abdominal) pain about 3 to 5 weeks after taking ella, you may have a pregnancy outside of the uterus (womb), which is called an ectopic or tubal pregnancy. An ectopic pregnancy is a serious condition that needs medical treatment right away. Call your healthcare provider or go to the nearest emergency room right away if you think you may have an ectopic pregnancy.
How often can I use ella?
ella is meant for emergency contraception only, and is not to be used frequently or as a regular birth control. If you need to use emergency contraception often, talk to your healthcare provider and learn about methods for birth control and sexually transmitted disease prevention that are right for you.
What are the possible side effects of ella?
The most common side effects of ella include:
- headache
- nausea
- stomach (abdominal) pain
- menstrual pain (dysmenorrhea)
- tiredness
- dizziness
Some women taking ella may have their next period earlier or later than expected. If your period is more than a week late, you should get a pregnancy test.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of ella. For more information, ask your healthcare provider or pharmacist.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA 1-800-FDA-1088.How should I store ella?
- Store ella at 68-77°F (20-25°C).
- Protect ella from light. Keep ella in the blister card inside the original box until you are ready to take it.
Do not use ella if the package is torn or broken.
Keep ella and all medicines out of the reach of children.General information about the safe and effective use of ella:
Medicines are sometimes prescribed for purposes other than those in a Patient Information Leaflet. Do not use ella for a condition for which it was not prescribed. Do not give ella to other people, even if they have the same symptoms that you have. It may harm them.
In the case of an overdose, get medical help or contact a Poison Control Center right away at 1-800-222-1222. Overdose experience with ella is limited.This Patient Information Leaflet summarizes the most important information about ella. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about ella that is written for health professionals.
For more information, go to www.ella-now.com or you can contact HRA Pharma America Inc, Health and Safety Team at 844-994-0329.
What are the ingredients in ella? Active ingredients: ulipristal acetate, 30 mg
Inactive ingredients: lactose monohydrate, povidone, croscarmellose sodium, and magnesium stearateFor all medical inquiries contact:
HRA Pharma America Inc.,
Morristown, NJ 07960Manufactured for :
HRA Pharma America Inc.,
Morristown, NJ 07960Under License From:
Laboratoire HRA Pharma, 92320 Chatillion, Franceella® is a registered trademark of Laboratoire HRA Pharma
Manufactured by:
Cenexi, 95520 Osny, France, or
Laboratorios León Farma S.A., 24008 León, Spain, or
Delpharm Lille SAS, 59452 Lys-Lez-Lannoy, FranceContent Updated: 11/2019
-
PRINCIPAL DISPLAY PANEL - 30 mg Tablet Blister Pack Carton
NDC 73302-456-01
ella®
ulipristal acetate
tablet 30 mg
HRAPharma
Rx only
Contains 1 Tablet
ella®
ulipristal acetate
tablet 30 mg
Each table contains
30 mg ulipristal acetate.
Usual dose: See package insert.
Take one tablet as soon as possible, within 5 days after unprotected intercourse.
Ella is contraindicated during pregnancy.
Store at 20-25°C (68-77°F). [See USP controlled room temperature.]
Keep the blister in the outer carton in order to protect from light.
Keep out of reach of children.
For Medical Emergencies - Contact HRA Pharma America Inc, Health and Safety Team at 844-994-0329
HRAPharma
Manufactured for
HRA Pharma America Inc.
36 Cattano Ave. Suite 400 Morristown,
NJ 07960
Under License From:
Laboratoire HRA Pharma
92330 Chatillon, France
Product of Spain
Manufactured By:
Delpharm Lille S.A.S.
PARC D'ACTIVITE ROUBAIX EST
22 RUE DE TOUFFLERS
CS 50070
59492 LYS LEZ LANNOY
FRANCE
5044762
ella®
ulipristal acetate table 30 mg
N 73302-456-1
Push through from front
Contains 1
30 mg tablet
Manufactured for:
HRA Pharma America Inc.,
36 Cattano Ave. Suite 400
Morristown, NJ 07960
LOT:
EXP:
5044761
-
INGREDIENTS AND APPEARANCE
ELLA
ulipristal acetate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 73302-456 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ULIPRISTAL ACETATE (UNII: YF7V70N02B) (ULIPRISTAL - UNII:6J5J15Q2X8) ULIPRISTAL ACETATE 30 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) POVIDONE K30 (UNII: U725QWY32X) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color WHITE (white to off-white) Score no score Shape ROUND Size 9mm Flavor Imprint Code ella Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73302-456-01 1 in 1 CARTON 05/11/2020 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022474 05/11/2020 Labeler - HRA Pharma America, Inc. (081160441) Registrant - Laboratoire HRA Pharma (502511996) Establishment Name Address ID/FEI Business Operations Delpharm Lille S.A.S. 283582547 manufacture(73302-456) , analysis(73302-456) , pack(73302-456) Establishment Name Address ID/FEI Business Operations CENEXI 263382610 manufacture(73302-456) , analysis(73302-456) , pack(73302-456) Establishment Name Address ID/FEI Business Operations Laboratorios Leon Farma S.A. 467782459 manufacture(73302-456) , analysis(73302-456) , pack(73302-456)
Trademark Results [Ella]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ELLA 98848129 not registered Live/Pending |
Sahakian, Taleen 2024-11-11 |
 ELLA 98825909 not registered Live/Pending |
JUSTRETAIL.AI,LLC 2024-10-29 |
 ELLA 98752902 not registered Live/Pending |
EI Innovations 2024-09-16 |
 ELLA 98413345 not registered Live/Pending |
Hirsch, Mark 2024-02-20 |
 ELLA 98355577 not registered Live/Pending |
Ella Mum, LLC 2024-01-12 |
 ELLA 98237670 not registered Live/Pending |
Empowerment Technologies Inc. 2023-10-24 |
 ELLA 98140438 not registered Live/Pending |
SHENZHEN TRANSCHAN TECHNOLOGY LIMITED 2023-08-18 |
 ELLA 98140412 not registered Live/Pending |
SHENZHEN TRANSCHAN TECHNOLOGY LIMITED 2023-08-18 |
 ELLA 97490340 not registered Live/Pending |
Gavriella R. Colton 2022-07-06 |
 ELLA 97444517 not registered Live/Pending |
Cool Coffee Holdings, LLC 2022-06-06 |
 ELLA 97444472 not registered Live/Pending |
Cool Coffee Holdings, LLC 2022-06-06 |
 ELLA 97296443 not registered Live/Pending |
Esellas LLC 2022-03-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.