ZYCORTAL- desoxycorticosterone pivalate injection, suspension
ZYCORTAL by
Drug Labeling and Warnings
ZYCORTAL by is a Animal medication manufactured, distributed, or labeled by Dechra Veterinary Products, Dechra Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- CAUTION:
-
DESCRIPTION:
Desoxycorticosterone pivalate is a mineralocorticoid hormone. Chemically, desoxycorticosterone pivalate is 21-(2,2-dimethyl-1-oxopropoxy)-pregn-4-ene-3,20-dione.
The structural formula is:
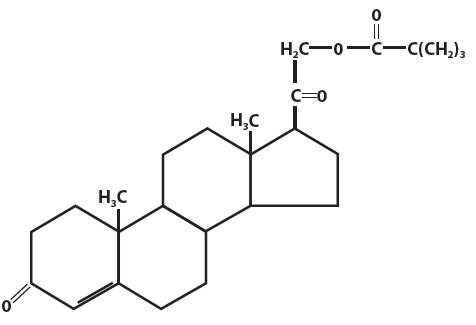
Molecular Formula: C26H38O4
ZYCORTAL Suspension is a white aqueous suspension.
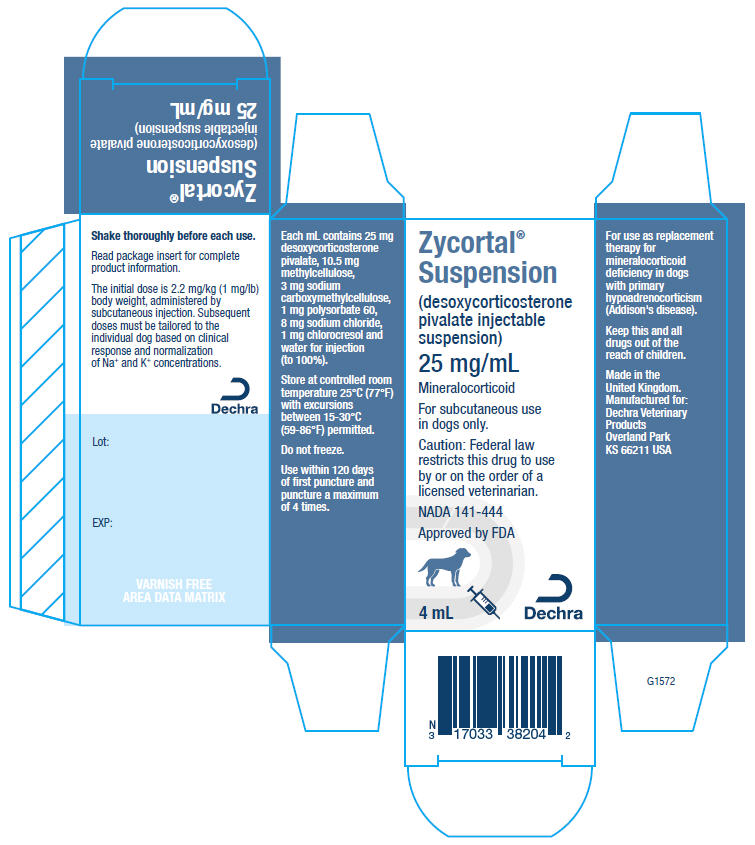
Each milliliter contains 25 mg of desoxycorticosterone pivalate. Inactive ingredients are 10.5 mg methylcellulose, 3 mg sodium carboxymethylcellulose, 1 mg polysorbate 60, 8 mg sodium chloride, 1 mg chlorocresol and water for injection (to 100%).
- INDICATION:
-
DOSAGE AND ADMINISTRATION:
Prior to each use, thoroughly shake the vial to resuspend the product.
ZYCORTAL Suspension replaces the mineralocorticoid hormones only. Dogs with combined glucocorticoid and mineralocorticoid deficiency should also be treated with prednisone or prednisolone at an initial dosage of 0.2-0.4 mg/kg/day (0.1-0.2 mg/lb/day).
ZYCORTAL Suspension is intended for long-term administration at intervals and doses dependent upon individual response. Tailor the dose of ZYCORTAL Suspension and the concurrently administered glucocorticoid replacement therapy to the individual dog based on clinical response and normalization of Na+ and K+ concentrations.
Initial dose of ZYCORTAL Suspension:
The initial dose is 2.2 mg/kg (1 mg/lb) body weight, administered by subcutaneous injection.
Interim monitoring visit:
Re-evaluate the dog and measure the serum sodium/potassium ratio (Na+/K+ ratio) approximately 10 days after the first dose, which is the time to maximum concentration (Tmax) of desoxycorticosterone (see CLINICAL PHARMACOLOGY). If the dog's clinical signs have worsened or not resolved, adjust the dose of prednisone/prednisolone and/or investigate other causes of the clinical signs.
Second dose of ZYCORTAL Suspension:
At approximately 25 days after the first dose, re-evaluate the dog and repeat the Na+/K+ ratio.
- If the dog is both clinically normal and has a normal Na+/K+ ratio on Day 25, adjust the dose based on the Day 10 Na+/K+ ratio using the guidelines in Table 1, below.
- If the dog is clinically normal and has a Na+/K+ ratio > 32 on Day 25, either adjust the dose based on the Day 10 Na+/K+ ratio according to Table 1 or delay the dose (see Prolonging the dosing interval).
- If the dog is either not clinically normal or if the Na+/K+ ratio is abnormal on Day 25, adjust the dose of prednisone/prednisolone or ZYCORTAL Suspension (see Subsequent doses and long-term management).
Table 1: Day 25: Administering the Second Dose of ZYCORTAL Suspension If the Day 10 Na+/K+ ratio is: Do not administer Dose 2 on Day 10. 25 days after the first dose, administer ZYCORTAL Suspension, as follows: > 34 Decrease dose to: 2.0 mg/kg > 32 to 34 Decrease dose to: 2.1 mg/kg 27 to 32 Continue 2.2 mg/kg 24 to < 27 Increase dose to: 2.3 mg/kg < 24 Increase dose to: 2.4 mg/kg Prolonging the dosing interval:
If the dog is clinically normal and the Day 25 Na+/K+ ratio is > 32, it is possible to prolong the dosing interval instead of adjusting the dose as described in Table 1. Evaluate the electrolytes every 3-7 days until the Na+/K+ ratio is < 32, and then administer 2.2 mg/kg of ZYCORTAL Suspension.
Subsequent doses and long-term management:
For subsequent doses, use the following guidelines if the dog is not clinically normal and/or has abnormal Na+ or K+ concentrations:
- Clinical signs of polyuria/polydipsia: Decrease the prednisone/prednisolone dose first. If the polyuria/polydipsia persists, then decrease the dose of ZYCORTAL Suspension without changing the dosing interval.
- Clinical signs of depression, lethargy, vomiting, diarrhea or weakness: Increase prednisone/prednisolone dose.
- Hyperkalemia, hyponatremia or Na+/K+ ratio < 27: Decrease the ZYCORTAL Suspension dosing interval by 2-3 days.
- Hypokalemia or hypernatremia: Decrease the ZYCORTAL Suspension dose.
Prior to a stressful situation, consider temporarily increasing the dose of prednisone/prednisolone.
- CONTRAINDICATIONS:
-
WARNINGS:
Use ZYCORTAL Suspension with caution in dogs with congestive heart disease, edema, severe renal disease or primary hepatic failure. Desoxycorticosterone pivalate may cause polyuria, polydipsia, increased blood volume, edema and cardiac enlargement. Excessive weight gain may indicate fluid retention secondary to sodium retention.
Do not use desoxycorticosterone pivalate in pregnant dogs.
- HUMAN WARNINGS:
-
PRECAUTIONS:
Any dog presenting with severe hypovolemia, dehydration, pre-renal azotemia and inadequate tissue perfusion ("Addisonian crisis") must be rehydrated with intravenous fluid (saline) therapy before starting treatment with ZYCORTAL Suspension.
The effectiveness of ZYCORTAL Suspension may be reduced if potassium-sparing diuretics, such as spironolactone, are administered concurrently.
-
ADVERSE REACTIONS:
One hundred fifty-two dogs were included in the field safety analysis. Adverse reactions are summarized in Table 2.
Table 2: Percentage of Dogs with Adverse Reactions in the Field Study Adverse Reaction ZYCORTAL Suspension
(n = 113 dogs)Active Control
(n = 39 dogs)Polyuria 15.0% (17) 12.8% (5) Polydipsia 13.3% (15) 15.4% (6) Depression/lethargy 9.7% (11) 2.6% (1) Inappropriate urination 8.0% (9) 10.3% (4) Alopecia 5.3% (6) 5.1% (2) Decreased appetite/anorexia 4.4% (5) 2.6% (1) Panting 3.5% (4) 0.0% (0) Vomiting 3.5% (4) 0.0% (0) Diarrhea 2.7% (3) 7.7% (3) Shaking/trembling 2.7% (3) 2.6% (1) Polyphagia 1.8% (2) 2.6% (1) Urinary tract infection 1.8% (2) 0.0% (0) Urinary incontinence 0.9% (1) 2.6% (1) Restlessness 0.9% (1) 2.6% (1) Urticaria/facial edema 0.0% (0) 5.1% (2) One dog with a pre-existing Grade III/VI heart murmur developed congestive heart failure 17 days after the first administration of ZYCORTAL Suspension and was removed from the study.
In addition to the adverse reactions reported during the field study, post-approval adverse drug experience reporting for desoxycorticosterone pivalate injectable suspension included reports of anaphylaxis and anemia.
To report suspected adverse events, for technical assistance or to obtain a copy of the safety data sheet (SDS), contact Dechra at (866) 933-2472.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at http://www.fda.gov/AnimalVeterinary/SafetyHealth.
-
CLINICAL PHARMACOLOGY:
Desoxycorticosterone is a corticosteroid with primarily mineralocorticoid activity, similar to aldosterone. In the kidney, desoxycorticosterone causes sodium and chloride ion retention, and hydrogen and potassium ion excretion, creating an osmotic gradient. The osmotic gradient promotes water absorption from the renal tubules resulting in increased extracellular fluid volume, leading to blood volume expansion, improved venous return to the heart, and increased cardiac output.
After subcutaneous administration of 11 mg/kg body weight (five times the labeled starting/initial dose of 2.2 mg/kg) of ZYCORTAL Suspension, the plasma half-life (mean ± standard deviation) is approximately 17 ± 7 days, with a maximum concentration (Cmax) of 13.2 ± 5 ng/mL, and time to maximum concentration (Tmax) of 10 ± 3.5 days.
-
EFFECTIVENESS:
A double-blinded, multi-site, 180-day field study evaluated the effectiveness of ZYCORTAL Suspension compared to an FDA-approved desoxycorticosterone pivalate active control. One hundred fifty-two (152) dogs of various breeds, 0.5-12.4 years of age and weighing 0.95-61.2 kg were enrolled. One hundred thirteen (113) dogs were treated with ZYCORTAL Suspension and 39 dogs were treated with the active control. Both groups were administered an initial dose of 2.2 mg/kg. Subsequent doses administered and/or frequency of administration were adjusted according to the clinical needs of the dog.
A dog was considered a treatment success if it remained clinically normal or had improved clinical signs compared to baseline and the Na+ and K+ concentrations were within the reference range of the analyzer, or the Na+/K+ ratio was between 27-32. Success rates for ZYCORTAL Suspension were 86.2% and 88.3% on Days 90 and 180, respectively; success rates for the active control were 85.1% and 86.9% on Days 90 and 180, respectively.
The mean final dose for ZYCORTAL Suspension was 1.9 ± 0.27 mg/kg (range 1.2-2.5 mg/kg) and the mean final dose interval was 38.5 ± 12.5 days (range 20-99 days) with the majority of dogs having a dosing interval between 20 and 46 days.
-
ANIMAL SAFETY:
In a laboratory study, ZYCORTAL Suspension was administered via subcutaneous injection to 32 Beagle dogs (four groups of 8 dogs each) at doses of 0, 1, 3 and 5 times the labeled starting dose (1× = 2.2 mg/kg), once every 21 days for 6 months, for a total of 9 injections. The volume injected in 3× and 5× dogs was equally divided between three and five sites, respectively. Dogs in the 1× group were dosed at a single injection site. Control dogs (0×) received subcutaneous injections of 0.9% sodium chloride at a volume equivalent to the 5× dose.
The most frequently noted abnormal clinical observations were injection site reactions in treated dogs, characterized by erythema and edema. Clinical pathology findings considered related to ZYCORTAL Suspension treatment included: decreased mean corpuscular volume in 3× and 5× groups; increased globulin concentrations in all treated groups; decreased potassium concentrations in all treated groups; increased sodium concentrations in all treated groups; decreased chloride concentrations in the 3× group; decreased blood urea nitrogen concentrations in all treated groups; and decreased urine specific gravity concentrations in all treated groups. Gross necropsy findings considered treatment-related included: subcapsular and cortical renal cysts, corresponding histologically with vascular tunica media hyperplasia; and irregular white plaques in the injection site subcutaneous tissue, corresponding histologically with granulomatous inflammation. Additional histology findings considered treatment-related included: chronic inflammation of the renal cortices, cortical tubular basophilia, cortical tubular dilation, glomerulopathy (3× and 5× groups), and adrenal gland vacuolation (zona glomerulosa).
- STORAGE INFORMATION:
- HOW SUPPLIED:
-
SPL UNCLASSIFIED SECTION
NADA 141-444, Approved by FDA
NDC: 17033-382-04
Manufactured for:
Dechra Veterinary Products
7015 College Boulevard, Suite 525
Overland Park, KS 66211
(866) 933-2472Manufactured in the United Kingdom.
© 2015 Dechra Ltd
ZYCORTAL is a trademark of Dechra Ltd; all rights reserved.F1194
- PRINCIPAL DISPLAY PANEL - 4 mL Vial Carton
-
INGREDIENTS AND APPEARANCE
ZYCORTAL
desoxycorticosterone pivalate injection, suspensionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 17033-382 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DESOXYCORTICOSTERONE PIVALATE (UNII: 16665T4A2X) (DESOXYCORTONE - UNII:40GP35YQ49) DESOXYCORTICOSTERONE PIVALATE 25 mg in 1 mL Inactive Ingredients Ingredient Name Strength METHYLCELLULOSE (1500 MPA.S) (UNII: P0NTE48364) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) POLYSORBATE 60 (UNII: CAL22UVI4M) SODIUM CHLORIDE (UNII: 451W47IQ8X) CHLOROCRESOL (UNII: 36W53O7109) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 17033-382-04 1 in 1 CARTON 1 4 mL in 1 VIAL, MULTI-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141444 03/15/2016 Labeler - Dechra Veterinary Products (362142734) Registrant - Dechra Ltd (641097493)
Trademark Results [ZYCORTAL]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ZYCORTAL 79153619 4697851 Live/Registered |
Dechra Limited 2014-06-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.