Careline Skin Guard by COSMOPHARM LTD. Careline Skin Guard
Careline Skin Guard by
Drug Labeling and Warnings
Careline Skin Guard by is a Otc medication manufactured, distributed, or labeled by COSMOPHARM LTD.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CARELINE SKIN GUARD
INVISIBLE SPF 50- homosalate,octocrylene,octisalate,avobenzone,oxybenzone spray
COSMOPHARM LTD.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Careline Skin Guard
Active ingredients
HOMOSALATE 10%
OCTOCRYLENE 10%
ETHYL SALICYLATE 5%
BUTYLBENZOYLMETHANE 5%
BENZOPHENONE-3 5%
Purpose
HOMOSALATE .....................................................Sunscreen
OCTOCRYLENE ........................................................Sunscreen
ETHYL SALICYLATE .................................................Sunscreen
BUTYLBENZOYLMETHANE ........................................Sunscreen
BENZOPHENONE-3 .....................................................Sunscreen
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (See directions) decreases the risk of cancer and early skin aging causes by the sun.
Warnings
For external use only.
Do not use
- on damaged or broken skin.
- if you have known sensitivities to any one of the ingredients.
Directions:
- Apply liberally to entire body 15 minutes before sun exposure.
- Reapply:
- At least every 2 hours
- Immediately after towel drying
- After 80 minutes of swimming or sweating
Children under 6 month - ask a doctor
Sun Protection Measures:
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- Limit your time in the sun, especially from 10 a.m. - 2 p.m.
- Wear long-sleeve shirts, pants, hats and sunglasses.
- Reapply at least every 2 hours.
- Use a water resistant sunscreen if swimming or sweating.
Inactive ingredients
Alcohol SD#40, Diethylhexyl 2,6 Naphthalate, Acrylates/Octylacrylamide Copolymer, Parfum, Astaxanthin, Tocopheryl Acetate (Vitamin E),Diethylhexyl Syringylidenemalonate, Caprylic/Capric Triglyceride, Alpha Isomethyl Ionone, Amyl Cinnamal, Benzyl Benzoate, Benzyl Salicylate, Butylphenyl Methylpropional, Citral, Citronellol, Geraniol, HexylCinnamal, Hydroxycitronellal, Hydroxyisohexyl-3- Cyclohexene Carboxaldehyde, Limonene, Linalool, Cinnamyl Alcohol, Containes Oxybenzone*.
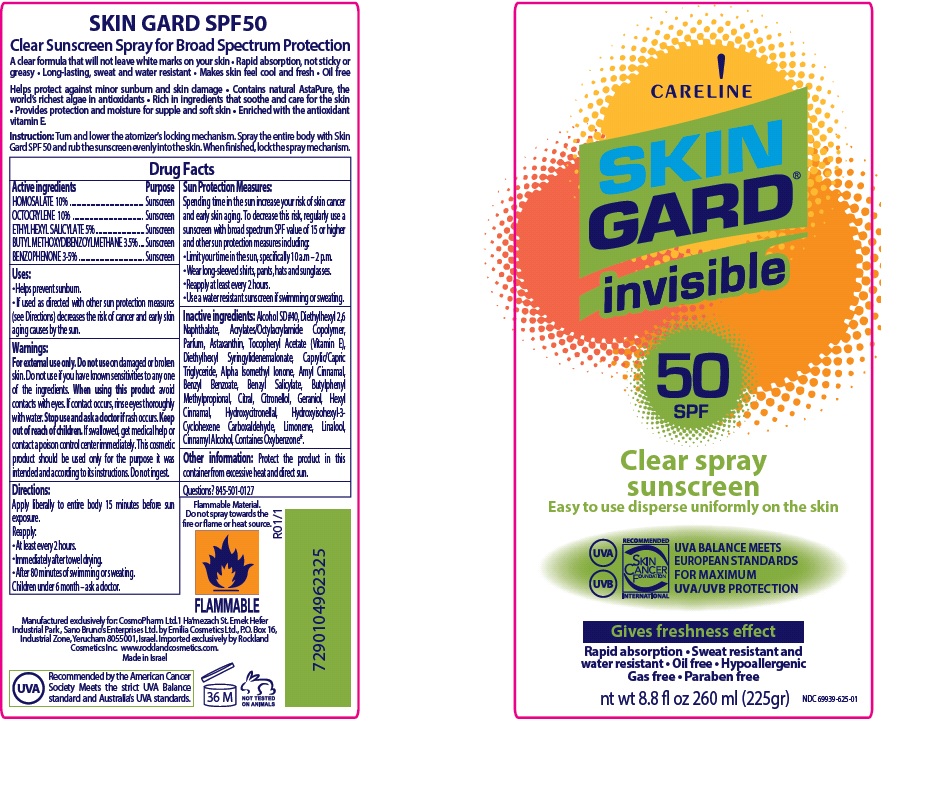
Principal Display Panel
Careline
SKIN GUARD
invisible
SPF 50
Clear spray sunscreen
Easy to use disperse uniformly on the skin
UVA UVB Protection
Gives freshness effect
Rapid absorption Sweat resistant and water resistant
Oil free Hypoallergenic
Gas free Paraben free
Net Wt. 8.8 fl oz. 260 ml (225 gr.)
NDC: 69939-625-01
| CARELINE SKIN GUARD
INVISIBLE SPF 50
homosalate,octocrylene,octisalate,avobenzone,oxybenzone spray |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - COSMOPHARM LTD. (600395487) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.