GRAFAPEX- treosulfan injection, powder, lyophilized, for solution
GRAFAPEX by
Drug Labeling and Warnings
GRAFAPEX by is a Prescription medication manufactured, distributed, or labeled by Medexus Pharma, Inc., Oncotec Pharma Produktion GmbH. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use GRAFAPEX™ safely and effectively. See full prescribing information for GRAFAPEX.
GRAFAPEX (treosulfan) for injection, for intravenous use
Initial U.S. Approval: 2025WARNING: MYELOSUPPRESSION
See full prescribing information for complete boxed warning.
- GRAFAPEX causes severe and prolonged myelosuppression.
- Hematopoietic stem cell transplantation is required to prevent potentially fatal complications of the prolonged myelosuppression. Monitor hematologic laboratory parameters. ( 5.1)
INDICATIONS AND USAGE
GRAFAPEX is an alkylating drug indicated for:
- Use in combination with fludarabine as a preparative regimen for allogeneic hematopoietic stem cell transplantation (alloHSCT) in adult and pediatric patients 1 year of age and older with acute myeloid leukemia (AML). ( 1.1).
- Use in combination with fludarabine as a preparative regimen for allogeneic hematopoietic stem cell transplantation in adult and pediatric patients 1 year of age and older with myelodysplastic syndrome (MDS). ( 1.2).
DOSAGE AND ADMINISTRATION
Recommended dosage: 10 g/m² body surface area (BSA) per day as a two hour intravenous infusion, given on three consecutive days (day -4, -3, -2) in conjunction with fludarabine before hematopoietic stem cell infusion (day 0). ( 2.1, 2.2)
- See Full Prescribing Information for instructions on preparation and administration. ( 2.2)
DOSAGE FORMS AND STRENGTHS
For injection: 1 g/vial and 5 g/vial treosulfan as a lyophilized powder in a single-dose vial. ( 3)
CONTRAINDICATIONS
- Hypersensitivity to any component of the drug product. ( 4)
WARNINGS AND PRECAUTIONS
- Seizures: Monitor signs of neurological adverse reactions and consider clonazepam prophylaxis for patients at higher risk. ( 5.2)
- Skin disorders: Keep skin clean and dry on days of GRAFAPEX infusion and change occlusive dressings after infusion. Change diapers frequently during the 12 hours after each infusion of GRAFAPEX. ( 5.3)
- Injection Site Reactions and Tissue Necrosis: May cause local tissue necrosis and injection site reactions, including erythema, pain, and swelling, in case of extravasation. If extravasation occurs, stop the infusion immediately and manage medically as required. ( 5.4)
- Secondary Malignancies: There is an increased risk of a secondary malignancy with use of GRAFAPEX. ( 5.5)
- Increased Early Morbidity and Mortality at Dosages Higher than Recommended: Avoid exceeding the recommended dosage of 10 g/m 2daily for three consecutive days. ( 5.6)
- Embryo-fetal toxicity: Can cause fetal harm. Advise patients of reproductive potential of the potential risk to a fetus and to use effective contraception. ( 5.7, 8.1, 8.3)
ADVERSE REACTIONS
The most common adverse reactions (≥20%) are musculoskeletal pain, stomatitis, pyrexia, nausea, edema, infection, and vomiting. ( 6.1) Selected Grade 3 or 4 nonhematological laboratory abnormalities are increased GGT, increased bilirubin, increased ALT, increased AST, and increased creatinine.
To report SUSPECTED ADVERSE REACTIONS, contact Medexus Pharma, Inc. at 1-855-336 -3322 or FDA at 1-800-FDA-1088 orwww.fda.gov/medwatch.
DRUG INTERACTIONS
- Certain CYP2C19 and CYP3A4 Substrates: Monitor for adverse reactions of these substrates where minimal concentration changes may lead to serious or life-threatening toxicities. ( 7.1)
USE IN SPECIFIC POPULATIONS
- Lactation: Advise not to breastfeed. ( 8.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 1/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: MYELOSUPPRESSION
1 INDICATIONS AND USAGE
1.1 Acute Myeloid Leukemia
1.2 Myelodysplastic Syndrome
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Preparation and Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Myelosuppression
5.2 Seizures
5.3 Skin Disorders
5.4 Injection Site Reactions and Tissue Necrosis
5.5 Secondary Malignancies
5.6 Increased Early Morbidity and Mortality at Dosages Higher than Recommended
5.7 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Effect of GRAFAPEX on Other Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: MYELOSUPPRESSION
- GRAFAPEX causes severe and prolonged myelosuppression at the recommended dosage.
- Hematopoietic stem cell transplantation is required to prevent potentially fatal complications of the prolonged myelosuppression. Monitor hematologic laboratory parameters[see Warnings and Precautions ( 5.1)] .
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dosage of GRAFAPEX is 10 g/m 2by intravenous infusion given daily for three days, beginning on Day -4 prior to transplantation in combination with fludarabine as outlined in Table 1.
Table 1: Dosage Regimen for GRAFAPEX-Based Allogeneic HSCT Treatment Day -6 Day -5 Day -4 Day-3 Day -2 Day -1 Day 0 GRAFAPEX 10 g/m 2/day intravenous infusion
X
X
X
Fludarabine 30 mg/m 2/day intravenous infusion
X
X
X
X
X
Allogeneic hematopoietic stem cell infusion
X
Premedicate patients with antiemetics prior to the first dose of GRAFAPEX and continue antiemetics on a fixed schedule through completion of treosulfan administration.
2.2 Preparation and Administration Instructions
Reconstitute GRAFAPEX prior to intravenous infusion.
GRAFAPEX is a hazardous drug. Follow applicable special handling and disposal procedures. 1
- Use aseptic technique to prepare GRAFAPEX.
- Calculate the dose, the total volume of reconstituted GRAFAPEX solution required, and the number of GRAFAPEX vials needed.
- Reconstitute each vial with 0.45% Sodium Chloride Injection, 0.9% Sodium Chloride Injection, 5% Dextrose Injection, or Sterile Water for Injection, in its original glass container using volumes described in Table 2 to obtain a final concentration of approximately 0.05 g/mL of GRAFAPEX. Reconstitution with Sterile Water for Injection alone is not recommended in children less than or equal to 12 years of age due to the resulting hypo-osmolarity of the final solution.
Table 2: Reconstitution Solution Volume Strength Volume 1 g/vial
20 mL
5 g/vial
100 mL
- Shake the vial(s) to dissolve.
- Inspect the reconstituted solution for discoloration and particulate matter. The reconstituted solution appears as a clear colorless solution. Solutions showing any sign of precipitation should not be used. In case that solubility issues occur, prolonged standing time or slight warming of the reconstituted solution (hand warm) may be useful.
- Determine the volume of 0.05 g/mL reconstituted solution needed based on the required dose. Reconstituted solutions of GRAFAPEX may be combined into a larger glass vial, ethylene-vinyl acetate (EVA) bag or polyolefin (PO) bag. Discard any unused portion left in the vial(s).
If not used immediately store reconstituted GRAFAPEX solution at room temperature 20°C to 25°C (68°F to 77°F) for up to 24 hours. Do not use if the solution contains a precipitate.
Do not refrigerate.
Infuse GRAFAPEX intravenously over 2 hours. Confirm patency of the intravenous line prior to infusion. Monitor for extravasation; if extravasation occurs, stop the infusion [see Warnings and Precautions ( 5.4)] .
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Myelosuppression
Profound myelosuppression with pancytopenia is the desired therapeutic effect of GRAFAPEX-based preparative regimens, occurring in all patients. Time to neutrophil counts > 0.5 Gi/L occurred at a median of 18 days (range 7-42 days) after allogeneic hematopoietic stem cell transplantation in adult patients treated using GRAFAPEX in combination with fludarabine as the preparative regimen.
Do not begin the preparative regimen if the stem cell donor is not available. Monitor blood cell counts daily until hematopoetic recovery. Provide standard supportive care for infections, anemia and thrombocytopenia until there is adequate hematopoietic recovery.
5.2 Seizures
There have been reports of seizures in patients following treatment with treosulfan. Monitor patients for signs of neurological adverse reactions. Clonazepam prophylaxis may be considered for patients at higher risk for seizures, including infants.
5.3 Skin Disorders
An increase of skin disorders (e.g. rash, dermatitis) was observed when patients received sodium bicarbonate-containing hydration in the course of treosulfan infusion, potentially because of acceleration of the pH‑dependent formation of alkylating epoxides [see Adverse Reactions ( 6.1) and Clinical Pharmacology ( 12.1)]. Keep skin clean and dry on days of GRAFAPEX infusion. Diaper dermatitis may occur because of excretion of treosulfan in the urine. Change diapers frequently during the 12 hours after each infusion of GRAFAPEX. Dermatitis may occur under occlusive dressings; change occlusive dressings after each infusion of GRAFAPEX.
5.4 Injection Site Reactions and Tissue Necrosis
GRAFAPEX may cause local tissue necrosis and injection site reactions, including erythema, pain, and swelling, in case of extravasation. Assure venous access patency prior to starting GRAFAPEX infusion, and monitor the intravenous infusion site for redness, swelling, pain, infection, and necrosis during and after administration of GRAFAPEX. If extravasation occurs, stop the infusion immediately and manage medically as required. Do not administer by the intramuscular or subcutaneous routes.
5.5 Secondary Malignancies
There is an increased risk of a secondary malignancy with use of GRAFAPEX. Treosulfan is carcinogenic and genotoxic [see Nonclinical Toxicology ( 13.1)] .
The risk of secondary malignancy is increased in patients with Fanconi anemia and other DNA breakage disorders. The safety and efficacy of GRAFAPEX have not been established for patients with these disorders.
5.6 Increased Early Morbidity and Mortality at Dosages Higher than Recommended
In MC‑FludT.14/L Trial I (NCT00822393), 330 adult patients were randomized to treosulfan at 14 g/m 2/day (1.4 times the recommended dose) for three consecutive days or busulfan at 3.2 mg/kg/day for two days, in combination with fludarabine as a preparative regimen for allogeneic transplantation. This trial was discontinued early due to a higher incidence of early fatal and/or serious adverse reactions in patients receiving treosulfan. Avoid exceeding the recommended GRAFAPEX dosage of 10 g/m 2daily for three consecutive days.
5.7 Embryo-Fetal Toxicity
Based on its mechanism of action, GRAFAPEX can cause fetal harm when administered to a pregnant woman because it is genotoxic and affects dividing cells [see Clinical Pharmacology ( 12) and Nonclinical Toxicology ( 13)]. Advise pregnant women of the potential risk to the fetus. Advise females of reproductive potential to use effective contraception during treatment with GRAFAPEX and for 6 months following the last dose of GRAFAPEX. Advise males with female partners of reproductive potential to use effective contraception during treatment with GRAFAPEX and for 3 months after the last dose [see Use in Specific Populations ( 8.1) and ( 8.3)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Myelosuppression [see Warnings and Precautions ( 5.1)]
- Seizures [see Warnings and Precautions ( 5.2)]
- Skin Disorders [see Warnings and Precautions ( 5.3)]
- Injection Site Reactions and Tissue Necrosis [see Warnings and Precautions ( 5.4)]
- Secondary Malignancies [see Warnings and Precautions ( 5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
MC‑FludT.14/L Trial II
The safety of GRAFAPEX was evaluated in 553 adult patients with AML and MDS in a randomized trial (MC‑FludT.14/L Trial II) comparing GRAFAPEX in combination with fludarabine to busulfan in combination with fludarabine as a preparative regimen for allogeneic hematopoietic stem cell transplantation. The patients were randomized to receive GRAFAPEX (n=270) 10 g/m² daily on day -4, -3 and -2 or to busulfan (n=283) 0.8 mg/kg every 6 hours on day -4 and -3 in combination with fludarabine 30 mg/m 2daily on day -6, -5, -4, -3 and -2, and hematopoietic stem cell transplantation on day 0 [see Clinical Studies ( 14)] .
Fatal regimen-related adverse reactions occurred within 30 days of transplantation in 1.9% of patients on the GRAFAPEX arm. All fatalities were due to pulmonary adverse reactions.
The most common adverse reactions (≥20%) in patients treated with GRAFAPEX were musculoskeletal pain, stomatitis, pyrexia, nausea, edema, infection, and vomiting. Selected Grade 3 or 4 nonhematological laboratory abnormalities were increased GGT, increased bilirubin, increased ALT, increased AST, and increased creatinine.
Table 3 shows the adverse reactions in Study MC‑FludT.14/L Trial II through transplant Day +30.
Table 3: Adverse Reactions in ≥10% of Patients through Transplant Day +30 in Study MC-FludT.14/L Trial II Adverse Reaction* All Grades Grades 3 or 4 GRAFAPEX(N = 270) Busulfan(N = 283) GRAFAPEX(N = 270) Busulfan(N = 283) % % % % Musculoskeletal pain 39 27 5 2 Stomatitis 38 48 6 7 Pyrexia 34 36 1 3 Nausea 33 41 3 6 Edema 29 18 0.7 1 Infection 1 23 18 12 6 Vomiting 22 19 1 1 Rash 17 13 1 1 Diarrhea 17 18 1 1 Headache 16 18 1 1 Febrile neutropenia 15 11 15 11 Abdominal pain 15 13 3 2 Hypertension 14 21 8 10 Hemorrhage 14 14 1 1 Fatigue 13 15 1 0.4 Constipation 12 12 0.4 0 Tachycardia 10 5 1 2 Hepatotoxicity 10 8 4 3 *Includes grouped terms
1Includes fatalities: n=6 in the GRAFAPEX arm and n=2 in the busulfan arm
Grading is based on Common Terminology Criteria for Adverse Events version 4.03Clinically relevant adverse reactions in <10% of patients who received GRAFAPEX included:
Neoplasms benign, malignant and unspecified (including cysts and polyps): second malignancy
Metabolism and nutrition disorders:
Decreased appetite, impaired glucose tolerance
Psychiatric disorders: Insomnia, confusional state, agitation
Nervous system disorders: Paresthesia, dizziness
Ear and labyrinth disorders: Vertigo
Cardiac disorders: Cardiac failure, pericardial effusion
Vascular disorders: Flushing, embolism, hypotension
Respiratory, thoracic and mediastinal disorders: Pneumonitis, pleural effusion, pharyngeal or laryngeal inflammation, dyspnea, cough, oropharyngeal pain, hiccups, dysphonia
Gastrointestinal disorders: Oral pain, gastritis, dyspepsia, dysphagia, abdominal distension, dry mouth
Skin and subcutaneous tissue disorders: Palmar plantar erythrodysesthesia syndrome, pruritus, erythema, dermatitis, skin hyperpigmentation, dry skin
Renal and urinary disorders: Acute kidney injury, hematuria, urinary tract pain
General disorders and administration site conditions: Chills, painInvestigations: Weight decreased or increased, increase of C‑reactive protein
All patients treated with GRAFAPEX and fludarabine developed neutropenia, anemia, and thrombocytopenia. One patient on the GRAFAPEX arm had graft failure. Table 4 summarizes the selected nonhematological laboratory abnormalities in Study MC-FludT.14/L Trial II by treatment arm through Day +30 posttransplant.
Table 4. Selected Grades 3-4 Laboratory Abnormalities through Transplant Day +30 in Study MC-FludT.14/L Trial II Laboratory Abnormality GRAFAPEX
N = 270 %Busulfan
N = 283 %Gamma Glutamyl Transferase Increased
16
28
Bilirubin Increased
6
5
Alanine Aminotransferase Increased
6
4
Aspartate Aminotransferase Increased
4
1
Creatinine Increased
3
0.7
Grading is based on Common Terminology Criteria for Adverse Events version 4.03
6.2 Postmarketing Experience
The following additional adverse reactions have been identified during post approval use of GRAFAPEX in preparative regimens prior to hematopoietic stem cell transplantation in adult and pediatric patients in other countries. As these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Metabolism and Nutrition Disorders: Acidosis
Nervous system disorders: Peripheral sensory neuropathy
Renal and urinary disorders: Renal failure
Immune system disorders: Hypersensitivity -
7 DRUG INTERACTIONS
7.1 Effect of GRAFAPEX on Other Drugs
Certain CYP2C19 and CYP3A4 Substrates
Monitor for adverse reactions of certain CYP2C19 or CYP3A4 substrates where minimal concentration changes may lead to serious or life-threatening toxicities, and reduce the dosage, as needed, if recommended in the prescribing information of these substrates.
Treosulfan is a CYP2C19 and CYP3A4 inhibitor [see Clinical Pharmacology ( 12)] . Concomitant use of GRAFAPEX is predicted to increase the exposure of CYP2C19 and CYP3A4 substrates based on a mechanistic understanding of treosulfan metabolism, which may increase the risk of their adverse reactions.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on its mechanism of action, GRAFAPEX can cause fetal harm when administered to a pregnant woman because it is genotoxic and affects dividing cells [see Clinical Pharmacology ( 12) and Nonclinical Toxicology ( 13)] . There are no available human clinical data on the use of treosulfan in pregnant women to support an estimation of a drug-associated risk. Specific embryo-fetal developmental toxicity studies with treosulfan in animals were not conducted. Advise pregnant women of the potential risk to a fetus [see Data].
In the U.S. general population, the estimated background risk of major birth defects is 2% – 4% and of miscarriage is 15% – 20% of clinically recognized pregnancies.
Data
Animal Data
Animal reproductive or developmental toxicity studies were not conducted with treosulfan. Treosulfan is genotoxic and is toxic to dividing cells, suggesting it can cause embryotoxicity and teratogenicity.
8.2 Lactation
Risk Summary
There is no data on the presence of treosulfan or its metabolites in human milk, the effects on the breastfed child, or on milk production. Because of the potential for serious adverse reactions in breastfed children, advise women not to breastfeed during treatment with GRAFAPEX and for 1 week after the last dose.
8.3 Females and Males of Reproductive Potential
Based on its mechanism of action, GRAFAPEX can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations ( 8.1)].
Pregnancy Testing
Conduct pregnancy testing in females of reproductive potential within 7 days prior to initiating therapy with GRAFAPEX.
Contraception
Females
Advise females of reproductive potential to use effective contraception during and up to 6 months after treatment with GRAFAPEX.
Males
Because of the potential for genotoxicity, advise males with female partners of reproductive potential to use effective contraception during treatment with GRAFAPEX and for 3 months after the last dose [see Nonclinical Toxicology ( 13.1)]
Infertility
Based on findings in animal studies, GRAFAPEX can impair fertility in females and males, and may cause temporary or permanent infertility [see Nonclinical Toxicology ( 13.1)] .
8.4 Pediatric Use
The safety and efficacy of GRAFAPEX as part of a preparative regimen prior to allogeneic hematopoietic stem cell transplant in pediatric patients 1 year of age and older with AML or MDS have been established based on evidence from an adequate and well-controlled study in adults, with additional pharmacokinetic and safety data in 111 pediatric patients, including 22 infants (1 month to < 2 years), 54 children (2 to < 12 years), and 35 adolescents (12 to < 17 years) [see Adverse Reactions ( 6.1), Clinical Pharmacology ( 12.3), and Clinical Studies ( 14)] . The incidence of hepatic and gastrointestinal adverse reactions was higher in pediatric patients than in adults.
Safety and effectiveness of GRAFAPEX as part of a preparative regimen prior to allogeneic hematopoietic stem cell transplant in pediatric patients with AML or MDS younger than 1 year of age have not been established.
Juvenile Animal Toxicity Data
Treatment of juvenile rats from postnatal day (PND) 10 to 35 with daily doses of 10, 50 or 100 mg/kg treosulfan (approximately 0.006, 0.03, 0.06-‑fold the human dose based on body surface area, BSA) generally resulted in findings comparable to those seen in adult animals. A delayed physical development indicated by decreased body weight, reduced relative organs weights, and delayed time point of vaginal opening were noted in the high-dosed rats. In a separate study, single intravenous administrations of 500 mg/kg treosulfan (0.3-‑fold the human dose based on BSA) to juvenile (PND 10) and young adult (PND 34 – 35) rats, treosulfan concentrations in brain tissue were low compared to plasma concentrations, but were approximately 2- to 3-fold higher in juvenile rats (4-6%) in comparison to young adults (2-3%).
8.5 Geriatric Use
Of the total number of GRAFAPEX-treated patients with AML or MDS in Study MC-FludT.14/L Trial II (n=270), 73 (27%) were 65 to 74 years of age, and none were 75 years of age and older [see Clinical Studies ( 14)] . No significant differences in safety or effectiveness were observed between these subjects and younger subjects.
- 10 OVERDOSAGE
-
11 DESCRIPTION
GRAFAPEX for injection contains treosulfan, an alkylating drug. Treosulfan is known chemically as L-‑threitol ‑1,4-‑dimethanesulfonate. Treosulfan is soluble in water (7% m/v) at 25ᴼC. Treosulfan is not hygroscopic.Treosulfan has the molecular formula C 6H 14O 8S 2and a molecular weight of 278.3 g/mole. Treosulfan has the following chemical structure:
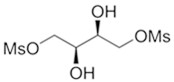
GRAFAPEX is intended for intravenous administration. It is supplied as a white, sterile, lyophilized powder for injection in glass vials containing 1 g or 5 g treosulfan.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Treosulfan is an alkylating agent. DNA alkylation is thought to be responsible for the cytotoxic activities of treosulfan. Treosulfan showed hematopoietic stem cell depleting activity as well as immunosuppressive and antitumor activity in mouse models of leukemia.
12.2 Pharmacodynamics
Increase in treosulfan exposure was associated with an increased incidence of infection-related death. Treosulfan time course of pharmacodynamic response is unknown.
12.3 Pharmacokinetics
Treosulfan pharmacokinetic parameters were observed at the approved recommended dosage, unless otherwise specified.
Treosulfan is a prodrug. Treosulfan mean ± SD area under the curve (AUC) is 1,200 ± 211 hr mcg/mL. There was no dose accumulation.
Distribution
Treosulfan mean (CV%) volume of distribution is approximately 41 liters (CV%: 19%). Treosulfan does not bind to plasma albumin.
Elimination
Treosulfan mean (± standard deviation) terminal half-life is 1.7 ± 0.4 hours.
Metabolism
The pharmacologically inactive treosulfan is converted spontaneously under physiological conditions into the active monoepoxide intermediate (2S,3S)‑1,2‑epoxybutane‑3,4‑diol‑4‑methanesulfonate) and finally to active L‑diepoxibutane (2S,3S)‑1,2:3,4‑diepoxybutane).
Excretion
A median of 42% of the treosulfan dose is excreted unchanged in the urine within 24 hours, and 89% of this unchanged fraction is excreted within the first 8 hours after administration.
Specific Populations
No clinically significant differences in the pharmacokinetics of treosulfan based on sex, mild renal impairment (CLcr 60‑89 mL/min), or mild hepatic impairment (total bilirubin less than or equal to ULN with AST greater than ULN or total bilirubin greater than 1 to 1.5 times ULN with any AST). The effect of moderate or severe renal impairment, moderate or severe hepatic impairment, and age ≥ 65 years on treosulfan pharmacokinetics is unknown.
Pediatric Patients
Treosulfan exposure in pediatric patients with BSA < 0.7 m 2and with BSA 0.7 to < 1.1 m 2are 11% and 5% higher, respectively, compared to adults.
No clinically significant difference in treosulfan median terminal half‑life was observed between pediatric patients and adults. The median terminal half‑life of the active monoepoxide intermediate was 1.6 hrs in pediatric patients.
Drug Interaction Studies
In Vitro Studies
CYP Enzymes:Treosulfan is a CYP2D6 substrate and its monoepoxide intermediate is a CYP2C8 substrate. Treosulfan inhibits CYP2C19 and CYP3A4 (using midazolam as substrate), but does not inhibit CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2D6, or CYP3A4 (using testosterone as substrate).
Transporter Systems: Treosulfan does not inhibit BCRP, BSEP, MATE1, OATP1B1, OATP1B3, OAT1, OAT3, OCT1, or OCT2.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis and Mutagenesis
No carcinogenicity study has been conducted. Treosulfan was mutagenic in the in vitro bacterial mutagenicity assay (Ames test) and clastogenic in the in vitro micronucleus assay in human lymphocytes.
No dedicated animal fertility studies were conducted. In a general toxicity study in rats, animals were treated with 5 or 50 mg/kg/day treosulfan orally for 7 months, 6 days a week. Spermatogenesis and ovarian function were significantly affected, starting at 5 mg/kg (0.003‑fold the human dose based on BSA). Findings included reduced weight of the testicles, seminal vesicle, prostate, and uterus, as well as spermatogenesis reduction and arrest, uterine atrophy, and reduced or absent corpora lutea and follicles.
-
14 CLINICAL STUDIES
The efficacy of GRAFAPEX was evaluated in a randomized active-controlled trial (MC‑FludT.14/L Trial II; NCT00822393) comparing GRAFAPEX to busulfan in combination with fludarabine as a preparative regimen for allogeneic transplantation. Eligible patients included adults 18 to 70 years old with acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS), Karnofsky performance status > 60%, and age ≥ 50 years or hematopoietic cell transplantation comorbidity index [HCT‑CI] score > 2. Patients were excluded if the creatinine clearance was < 60 mL/min, forced expiratory volume (FEV1) < 50% or on supplemental oxygen, left ventricular ejection fraction (LVEF) < 40%, bilirubin > 3X ULN, or aminotransferases (ALT or AST) > 5X ULN.
The patients were randomized to receive GRAFAPEX 10 g/m² daily on day -4, -3 and -2 or to busulfan 0.8 mg/kg every 6 hours on day -4 and -3 in combination with fludarabine 30 mg/m 2daily on day -6, -5, -4, -3 and -2, and hematopoietic stem cell transplantation on day 0. For the subset of patients with unrelated donors, antithymocyte globulin was administered in 97% and 95% of patients on each arm, respectively. Cyclosporine and methotrexate was used as graft‑vs‑host disease prophylaxis.
There were 570 patients randomized to GRAFAPEX (n = 280) or busulfan (n = 290). The efficacy population included 365 patients with AML and 205 patients with MDS: 536 patients received peripheral blood stem cells, 15 patients received marrow stem cells, and 19 patients were not transplanted. Table 5 shows the baseline characteristics of the study patients.
Table 5: Baseline characteristics of the study patients (MC‑FludT.14/L Trial II) Baseline Characteristics GRAFAPEX (n = 280) Busulfan (n = 290) Age, median (range), years
60.0 (37, 70)
60.5 (31, 70)
Age, n (%)
- 18-64 years
- 65-70 years
204 (73%)76 (27%)
218 (75%)
72 (25%)
Gender, n (%)
- Male
- Female
171 (61%)
109 (39%)176 (61%)
114 (39%)Weight, median (range), kg
80.0 (48.0, 144.0)
78.1 (46.0, 141.9)
Disease, n (%)
AML
MDS
192 (69%)
88 (31%)
173 (60%)
117 (40%)
AML remission status, n (%)
- CR1
- >CR1
164 (85%)
28 (15%)148 (86%)
25 (14%)MDS risk group, n (%) - Lower risk
- Higher risk
20 (23%)
68 (77%)20 (17%)
97 (83%)HCT‑CI score
- ≤2
- >2
116 (41%)
164 (59%)118 (41%)
172 (59%)Donor
- Matched related
- Matched unrelated
66 (24%)
214 (76%)68 (23%)
222 (77%)Abbreviations: AML: acute myeloid leukemia; MDS: myelodysplastic syndrome; CR1: complete remission 1; HCT-CI: hematopoietic cell transplant-specific comorbidity index
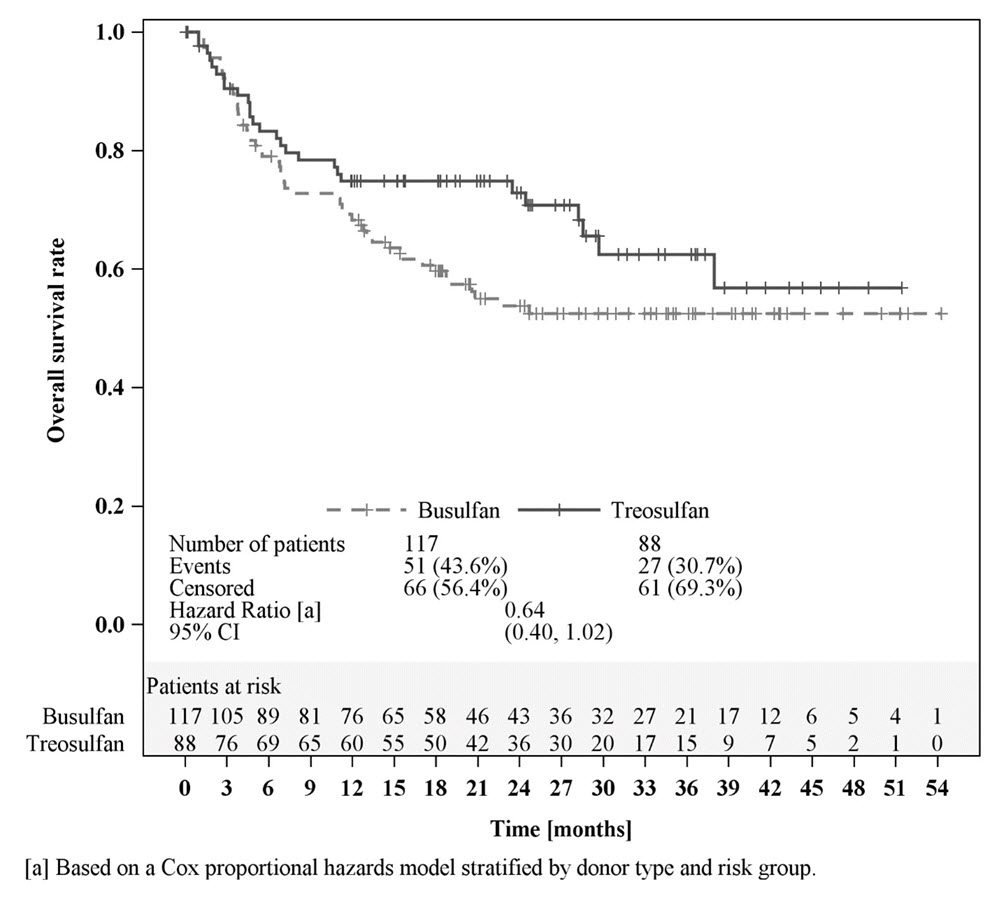
Efficacy was established on the basis of overall survival (OS), defined as the time from randomization until death from any cause. The hazard ratio (HR) for OS (stratified by donor type and risk group) compared to busulfan was 0.67 (95% CI: 0.51, 0.90) in the randomized population, 0.73 (95% CI: 0.51, 1.06) in patients with AML, and 0.64 (95% CI: 0.40, 1.02) in patients with MDS. Results are displayed in Figures 1, 2, and 3 below.
Figure 1: Kaplan-Meier estimates of overall survival since time of randomization MC FludT.14/L Trial II
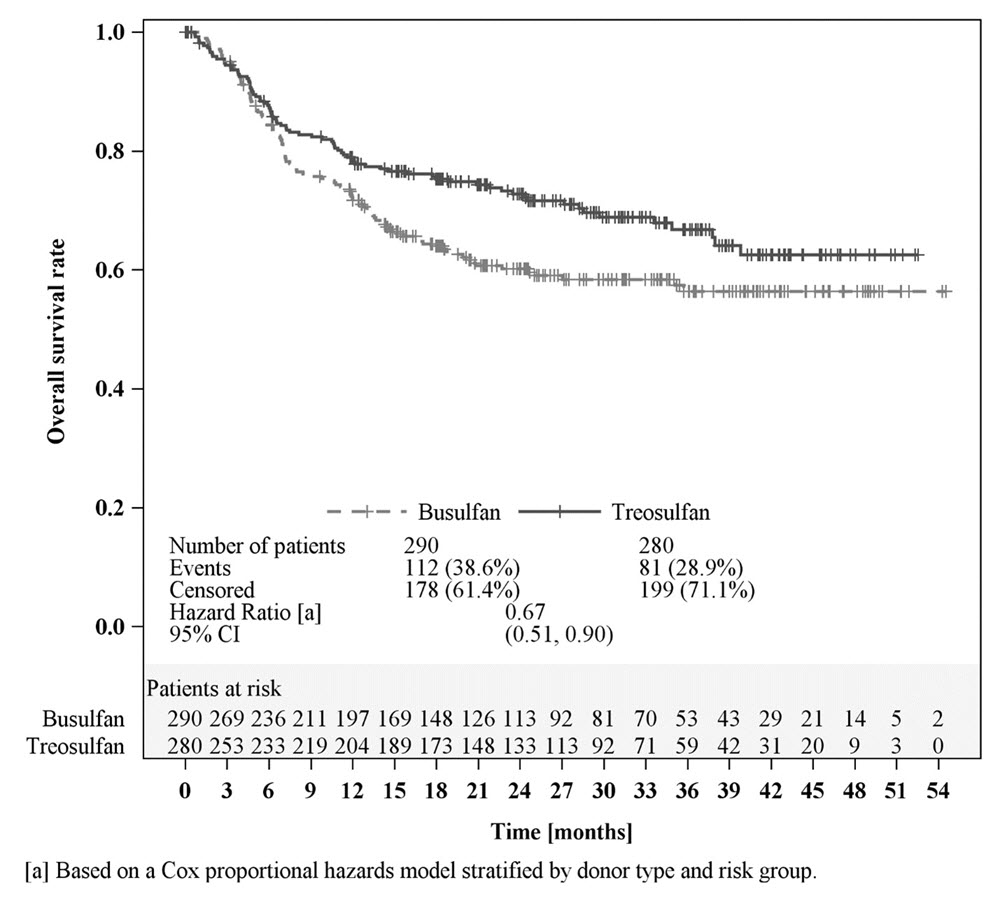
Figure 2: Kaplan-Meier estimates of overall survival since time of randomization MC FludT.14/L Trial II (patients with AML)
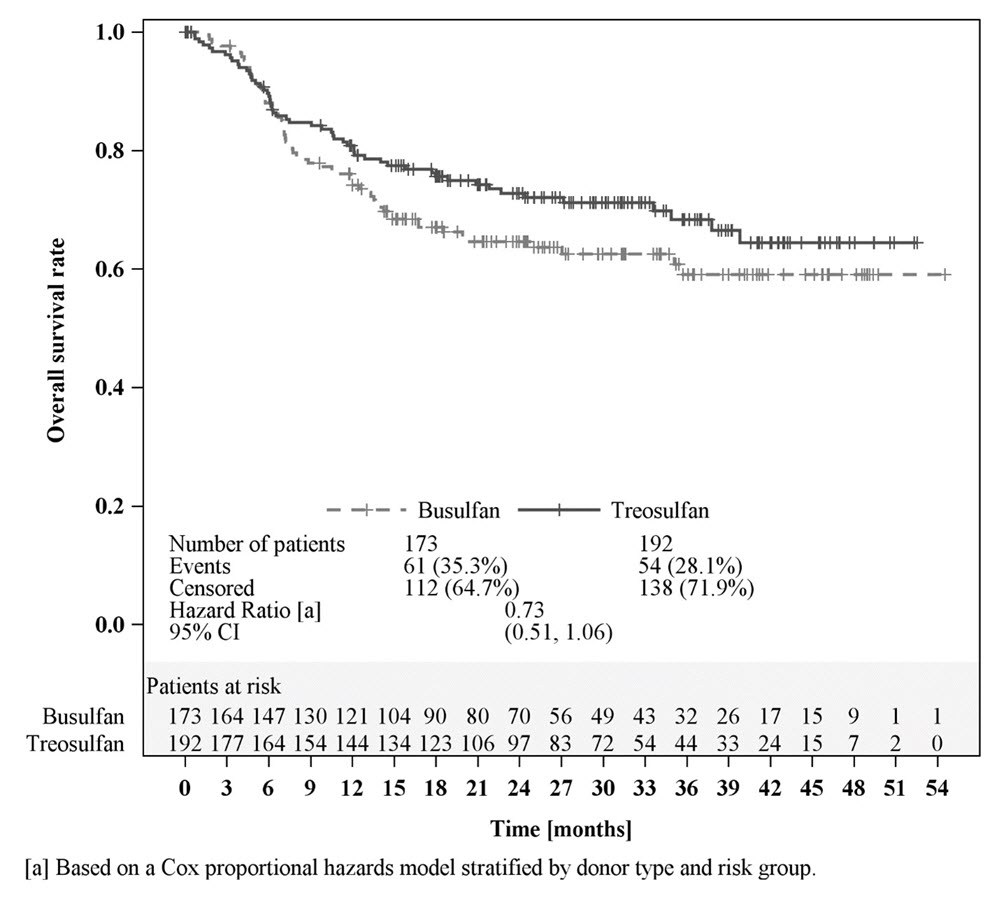
Figure 3: Kaplan-Meier estimates of overall survival since time of randomization MC FludT.14/L Trial II (patients with MDS)
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
GRAFAPEX (treosulfan) for injection is a white, sterile, lyophilized powder for reconstitution. It is supplied in a carton containing one single-dose vial.
Presentation
NDC
1 g/vial
59137-335-01
5 g/vial
59137-365-01
Storage and Handling
Store at 20°C to 25°C (68°F to 77°F) [see USP Controlled Room Temperature]. (Excursions permitted between 15°C and 30°C)
GRAFAPEX is a hazardous drug. Follow applicable special handling and disposal procedures 1.
-
17 PATIENT COUNSELING INFORMATION
Infections
- Inform patients of the increased risk of infections after treatment with GRAFAPEX that may require antibiotic, antiviral, or antifungal treatment and hospitalization.
- Advise patients to contact their healthcare provider immediately in case of any new or worsening signs of infection, e.g., cough, headache, diarrhea, or fever [see Warnings and Precautions ( 5.1)] .
Seizures
- Inform patients that seizures may occur [see Warnings and Precautions ( 5.2)] .
Skin Disorders
- Advise patients to clean “sweaty” skin parts (armpit, groin, genital area, inframammary line), each with a disposable washcloth and clear water.
- Advise patients not to apply any cream to the skin on the days of chemotherapy, and clothing should not be too tight, in order to let the skin “breathe” [see Warnings and Precautions ( 5.3)] .
Secondary Malignancies
- Inform patients of the possible risk of a second malignancy [see Warnings and Precautions ( 5.5)] .
Embryo-Fetal Toxicity
- Advise females of reproductive potential of the potential risk to a fetus and to inform their healthcare provider of a known or suspected pregnancy [see Use in Specific Populations ( 8.1)] .
- Advise females of reproductive potential to use effective contraception during treatment with GRAFAPEX and for 6 months following the last dose of GRAFAPEX [see Warnings and Precautions ( 5.7) and Use in Specific Populations ( 8.3)] .
- Advise males with female partners of reproductive potential to use effective contraception during treatment with GRAFAPEX and for 3 months after the last dose [see Warnings and Precautions ( 5.7) and Use in Specific Populations ( 8.3)] .
Lactation
- Advise women not to breastfeed during treatment with GRAFAPEX and for 1 week after the last dose [see Use in Specific Populations ( 8.2)].
Infertility
- GRAFAPEX can impair fertility in females and males, and may cause temporary or permanent infertility [see Use in Specific Populations ( 8.3)] .
Manufactured for:
Medexus Pharma, Inc.
Chicago, Illinois 60606
USAGRAFAPEX™ is a trademark of medac GmbH, Germany.
-
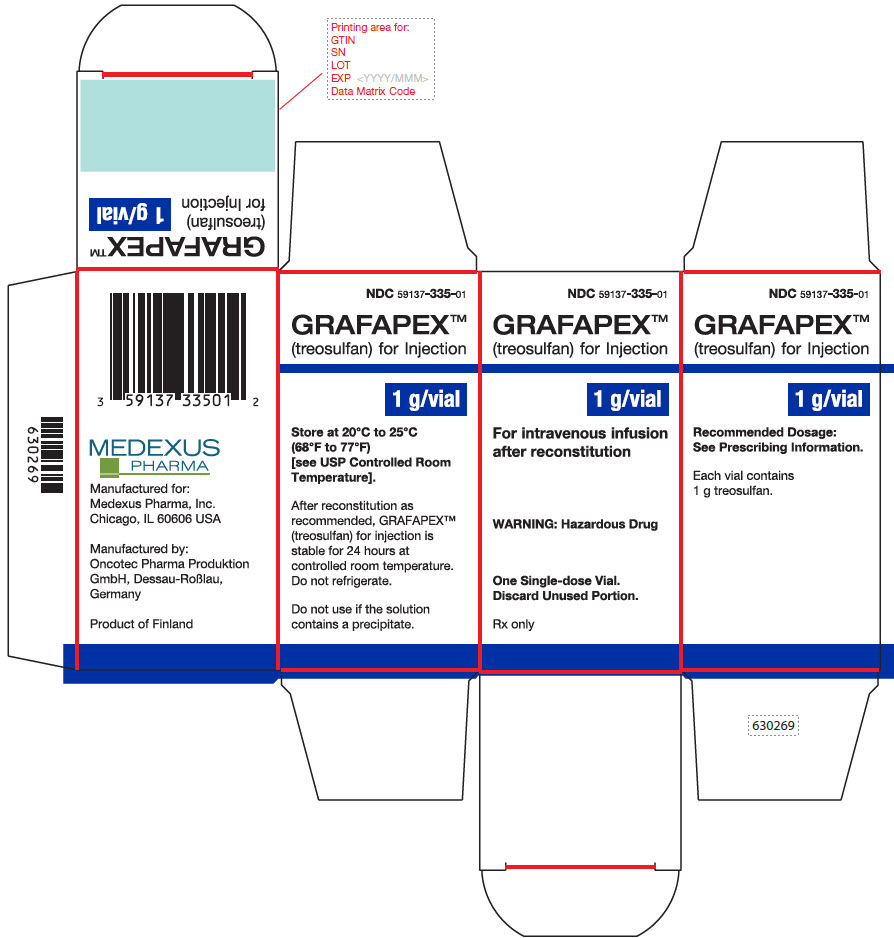
PRINCIPAL DISPLAY PANEL - GRAFAPEX™ 1 g
Carton Label - GRAFAPEX™ 1 g
NDC 59137- 335-01
GRAFAPEX™
(treosulfan) for injection
1 g/vialFor intravenous infusion
after reconstitutionWARNING: Hazardous Drug
One Single-dose Vial.
Discard Unused Portion.Rx only
One Single-dose Vial
Vial Label - GRAFAPEX™ 1 g
NDC59137 -335-00
GRAFAPEX™
(treosulfan)
for injection
1 g/vialFor intravenous infusion
after reconstitutionWARNING: Hazardous Drug
Single-dose Vial.
Discard Unused Portion.Recommended dosage: See Prescribing
Information. Store at 20°C to 25°C (68°F to
77°F) [see USP Controlled Room Temperature].
Do not use if solution contains a precipitate.Rx only
Manufactured for: Medexus Pharma, Inc.
Chicago, IL 60606 USAManufactured by:
Oncotec Pharma Produktion GmbH,
Dessau-Roβlau,
Germany
-
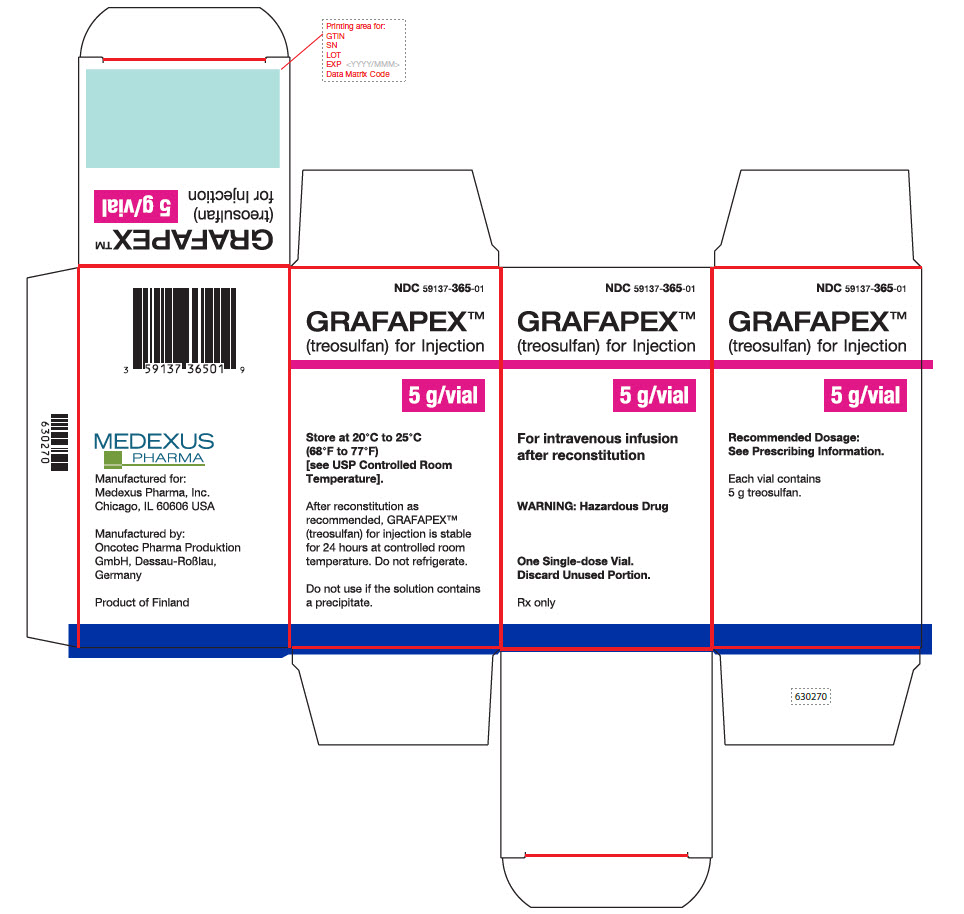
PRINCIPAL DISPLAY PANEL - GRAFAPEX™ 5 g
Carton Label - GRAFAPEX™ 5 g
NDC59137- 365-01
GRAFAPEX™
(treosulfan) for injection
5 g/vialFor intravenous infusion
after reconstitiutionWARNING: Hazardous Drug
One Single-dose Vial.
Discard Unused Portion.Rx only
Vial Label - GRAFAPEX™ 5 g
NDC59137- 365-00
GRAFAPEX™
(treosulfan)
for injection
5 g/vialFor intravenous infusionafter reconstitution
WARNING: Hazardous Drug
Single-dose Vial.
Discard Unused Portion.Recommended dosage: See Prescribing
Information. Store at 20°C to 25°C (68°F to
77°F) [see USP Controlled Room Temperature].
Do not use if solution contains a precipitate.Rx only
Manufactured for: Medexus Pharma, Inc.
Chicago, IL 60606 USAManufactured by:
Oncotec Pharma Produktion GmbH,
Dessau-Roβlau, Germany
-
INGREDIENTS AND APPEARANCE
GRAFAPEX
treosulfan injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 59137-335 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TREOSULFAN (UNII: CO61ER3EPI) (TREOSULFAN - UNII:CO61ER3EPI) TREOSULFAN 1000 mg in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59137-335-01 1 in 1 CARTON 01/28/2025 1 1 g in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214759 01/28/2025 GRAFAPEX
treosulfan injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 59137-365 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TREOSULFAN (UNII: CO61ER3EPI) (TREOSULFAN - UNII:CO61ER3EPI) TREOSULFAN 5000 mg in 5 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59137-365-01 1 in 1 CARTON 01/28/2025 1 5 g in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214759 01/28/2025 Labeler - Medexus Pharma, Inc. (078811131) Establishment Name Address ID/FEI Business Operations Oncotec Pharma Produktion GmbH 366308617 manufacture(59137-335, 59137-365) , analysis(59137-335, 59137-365)
Trademark Results [GRAFAPEX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 GRAFAPEX 98888869 not registered Live/Pending |
medac Gesellschaft für klinische Spezialpräparate mbH 2024-12-06 |
 GRAFAPEX 90491908 not registered Live/Pending |
medac Gesellschaft für klinische Spezialpräparate mbH 2021-01-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.