Atamel Forte by RAPHA HEALTH NETWORK INTERNATIONAL INC Atamel Forte
Atamel Forte by
Drug Labeling and Warnings
Atamel Forte by is a Otc medication manufactured, distributed, or labeled by RAPHA HEALTH NETWORK INTERNATIONAL INC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
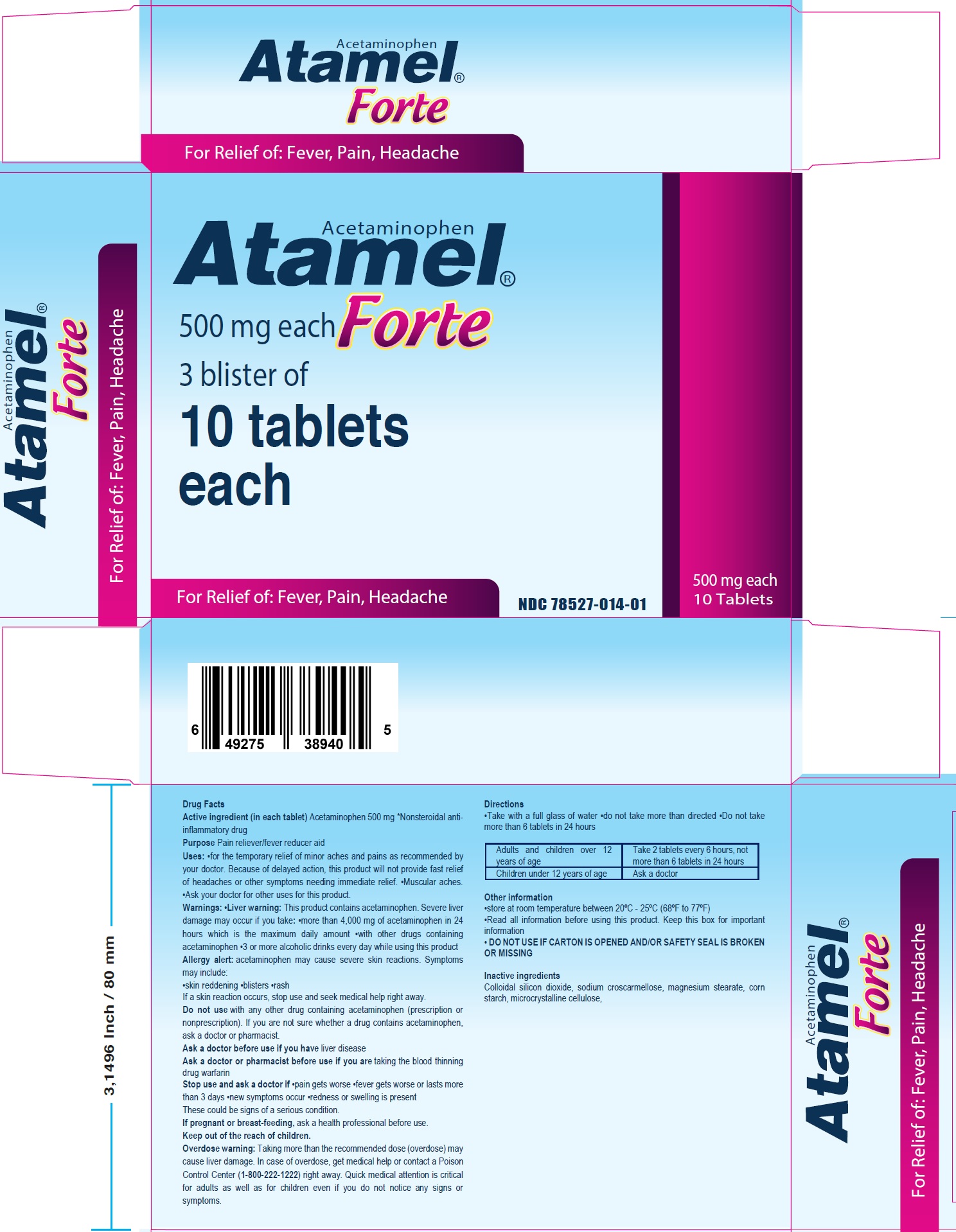
ATAMEL FORTE- acetaminophen tablet
RAPHA HEALTH NETWORK INTERNATIONAL INC
----------
Atamel Forte
Uses:
for the temporary relief of minor aches and pains as recommended by your doctor. Because of delayed action, this product will not provide fast relief of headaches or other symptoms needing immediate relief. Muscular aches. Ask your doctor for other uses for this product.
Warnings:
This product contains acetaminophen. Severe liver damage may occur if you take: more than 4,000 mg of acetaminophen in 24 hours which is the maximum daily amount with other drugs containing acetaminophen 3 or more alcoholic drinks every day while using this product acetaminophen may cause severe skin reactions. Symptoms may include: skin reddening blisters rash If a skin reaction occurs, stop use and seek medical help right away.
Liver warning:Allergy alert:
Do not use
with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
Stop use and ask a doctor if
pain gets worse fever gets worse or lasts more than 3 days new symptoms occur redness or swelling is present These could be signs of a serious condition.
Keep out of the reach of children.
Overdose warning: Taking more than the recommended dose (overdose) may cause liver damage. In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away. Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Directions
Take with a full glass of water do not take more than directed Do not take more than 6 tablets in 24 hours
| Adults and children over 12 years of age | Take 2 tablets every 6 hours, not more than 6 tablets in 24 hours |
| Children under 12 years of age | Ask a doctor |
Other information
store at room temperature between 20ºC - 25ºC (68ºF to 77ºF) Read all information before using this product. Keep this box for important information
DO NOT USE IF CARTON IS OPENED AND/OR SAFETY SEAL IS BROKEN OR MISSING
| ATAMEL FORTE
acetaminophen tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - RAPHA HEALTH NETWORK INTERNATIONAL INC (111018998) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| RAPHA HEALTH NETWORK INTERNATIONAL INC | 111018998 | manufacture(78527-014) | |