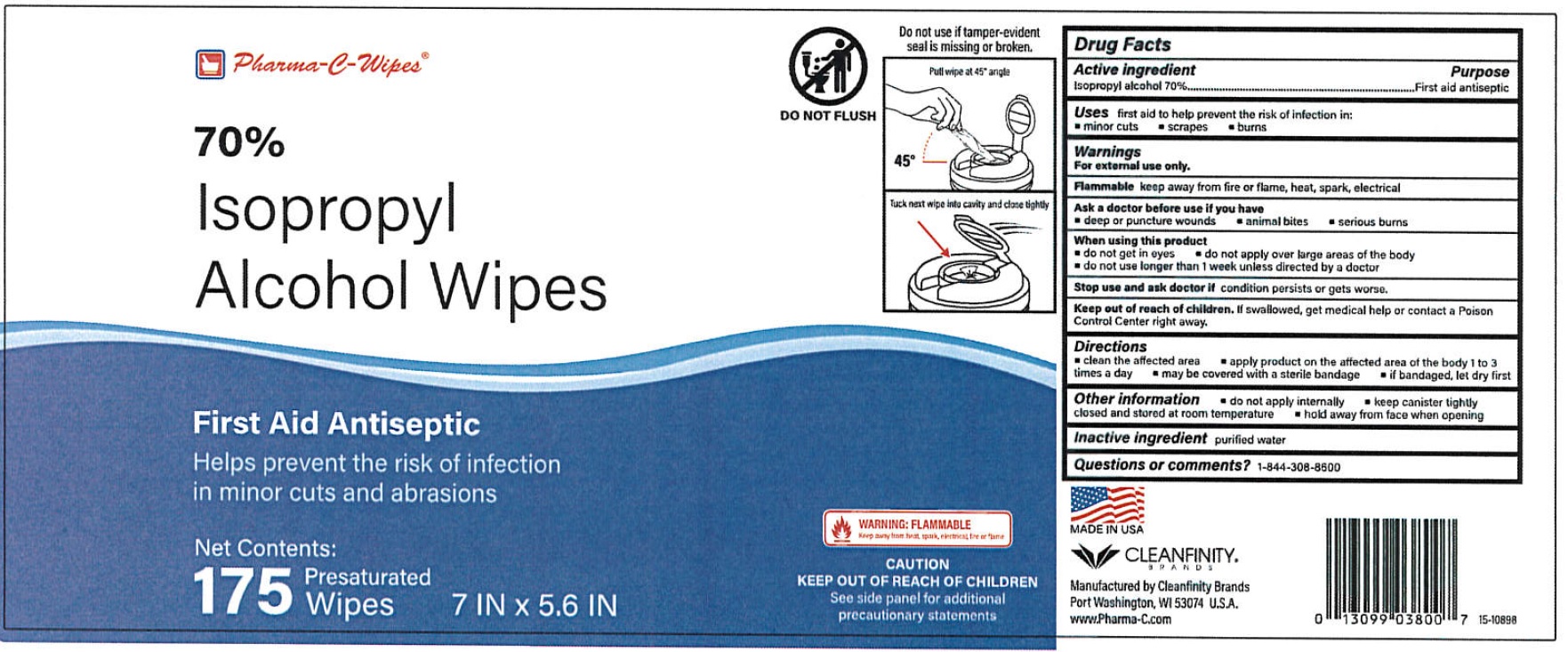
Pharma C Wipes 70% Isopropyl Alcohol Wipes
Pharma C Wipes 70 Isopropyl Alcohol Wipes by
Drug Labeling and Warnings
Pharma C Wipes 70 Isopropyl Alcohol Wipes by is a Otc medication manufactured, distributed, or labeled by Kleen Test Products Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
PHARMA C WIPES 70 ISOPROPYL ALCOHOL WIPES- isopropyl alcohol cloth
Kleen Test Products Corporation
----------
Pharma C Wipes 70% Isopropyl Alcohol Wipes
Warnings
For external use only
keep away from fire or flame, heat, spark, electrical Flammable
Directions
clean the affected area apply product on the affected area of the body 1 to 3 times a day may be covered with a sterile bandage if bandaged, let dry first
| PHARMA C WIPES 70 ISOPROPYL ALCOHOL WIPES
isopropyl alcohol cloth |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Kleen Test Products Corporation (168165814) |
Revised: 11/2023
Document Id: 0a09699a-0fa6-7404-e063-6394a90afebc
Set id: 2d19beec-eaa1-41e1-b277-2e0ebae832ee
Version: 3
Effective Time: 20231113