VAXCHORA- cholera vaccine, live, oral kit
Vaxchora by
Drug Labeling and Warnings
Vaxchora by is a Other medication manufactured, distributed, or labeled by Bavarian Nordic A/S, Bavarian Nordic Berna GmbH. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use VAXCHORA safely and effectively. See full prescribing information for VAXCHORA.
VAXCHORA® (Cholera Vaccine, Live, Oral)
Suspension for Oral Administration
Initial U.S. Approval: 2016INDICATIONS AND USAGE
VAXCHORA is a vaccine indicated for active immunization against disease caused by Vibrio cholerae serogroup O1. VAXCHORA is approved for use in persons 2 through 64 years of age traveling to cholera-affected areas. (1)
Limitations of Use:
- The effectiveness of VAXCHORA has not been established in persons living in cholera-affected areas. (1.1)
- The effectiveness of VAXCHORA has not been established in persons who have pre-existing immunity due to previous exposure to V. cholerae or receipt of a cholera vaccine. (1.1)
- VAXCHORA has not been shown to protect against disease caused by V. cholerae serogroup O139 or other non-O1 serogroups. (1.1)
DOSAGE AND ADMINISTRATION
- For oral administration only.
- Prepare and administer VAXCHORA in a healthcare setting equipped to dispose of medical waste. (2.3)
- Prepare VAXCHORA by reconstituting the buffer component in 100 milliliters (mL) of bottled water (purified, spring, or sparkling [carbonated]); for children 2 through 5 years of age, discard half the reconstituted buffer solution; then add the active component (lyophilized V. cholerae CVD 103-HgR). (2.3) After preparation, a single dose of VAXCHORA is 100 mL for persons 6 through 64 years or 50 mL for children 2 through 5 years of age. (3)
- Instruct recipients to avoid eating or drinking for 60 minutes before and after oral ingestion of VAXCHORA. (2.2)
- Administer VAXCHORA a minimum of 10 days before potential exposure to cholera. (2.1)
DOSAGE FORMS AND STRENGTHS
Suspension for oral administration supplied as a packet of the buffer component and a packet of the active component (lyophilized V. cholerae CVD 103-HgR). After preparation, a single dose of VAXCHORA is 100 mL for persons 6 years through 64 years of age or 50 mL for children 2 through 5 years of age. (3)
CONTRAINDICATIONS
Severe allergic reaction (e.g., anaphylaxis) to any ingredient of VAXCHORA or to a previous dose of any cholera vaccine. (4)
WARNINGS AND PRECAUTIONS
- The safety and effectiveness of VAXCHORA have not been established in immunocompromised persons. (5.1)
- VAXCHORA may be shed in the stool of recipients for at least 7 days. There is a potential for transmission of the vaccine strain to non-vaccinated close contacts (e.g., household contacts). Use caution when considering whether to administer VAXCHORA to individuals with immunocompromised close contacts. (5.2)
ADVERSE REACTIONS
The most common adverse reactions for adults (incidence > 3%) were tiredness (31%), headache (29%), abdominal pain (19%), nausea/vomiting (18%), lack of appetite (17%) and diarrhea (4%). (6)
The most common adverse reactions for children and adolescents (incidence ≥10%) were:
- Age 12 to <18 years: headache (45%), tiredness (41%), abdominal pain (38%), lack of appetite (29%) and nausea (22%).
- Age 6 to <12 years: tiredness (35%), abdominal pain (27%), headache (26%), lack of appetite (15%) and nausea (14%).
- Age 2 to <6 years: tiredness (31%), loss of appetite (19%), and abdominal pain (17%). (6)
To report SUSPECTED ADVERSE REACTIONS, contact Bavarian Nordic A/S at 1-833-365-9596 or VAERS at 1-800-822-7967 or www.vaers.hhs.gov.
DRUG INTERACTIONS
Avoid concomitant administration of VAXCHORA with systemic antibiotics since these agents may be active against the vaccine strain. Do not administer VAXCHORA to patients who have received oral or parenteral antibiotics within 14 days prior to vaccination. (7.2)
Immune responses to VAXCHORA may be diminished when VAXCHORA is administered concomitantly with chloroquine. Administer VAXCHORA at least 10 days before beginning antimalarial prophylaxis with chloroquine. (7.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 2/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Limitations of Use
2 DOSAGE AND ADMINISTRATION
2.1 Dose and Schedule
2.2 Restrictions on Eating and Drinking
2.3 Preparation, Reconstitution and Administration
2.4 Disposal Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Altered Immunocompetence
5.2 Shedding and Transmission
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Food and Drink
7.2 Concomitant Vaccines or Medications
7.3 Immunosuppressive Treatments
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Immunocompromised Individuals
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Efficacy Against V. cholerae Challenge
14.2 Immunogenicity
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
VAXCHORA is a vaccine indicated for active immunization against disease caused by Vibrio cholerae serogroup O1 in persons 2 through 64 years of age traveling to cholera-affected areas.
1.1 Limitations of Use
The effectiveness of VAXCHORA has not been established in persons living in cholera-affected areas.
The effectiveness of VAXCHORA has not been established in persons who have pre-existing immunity due to previous exposure to V. cholerae or receipt of a cholera vaccine.
VAXCHORA has not been shown to protect against disease caused by V. cholerae serogroup O139 or other non-O1 serogroups.
-
2 DOSAGE AND ADMINISTRATION
For oral administration only.
2.1 Dose and Schedule
Administer a single oral dose of VAXCHORA a minimum of 10 days before potential exposure to cholera.
The safety and effectiveness of revaccination with VAXCHORA have not been established.
2.2 Restrictions on Eating and Drinking
Instruct recipients to avoid eating or drinking for 60 minutes before and after oral ingestion of VAXCHORA.
2.3 Preparation, Reconstitution and Administration
Prepare and administer VAXCHORA in a healthcare setting equipped to dispose of medical waste [see Disposal Instructions (2.4)].
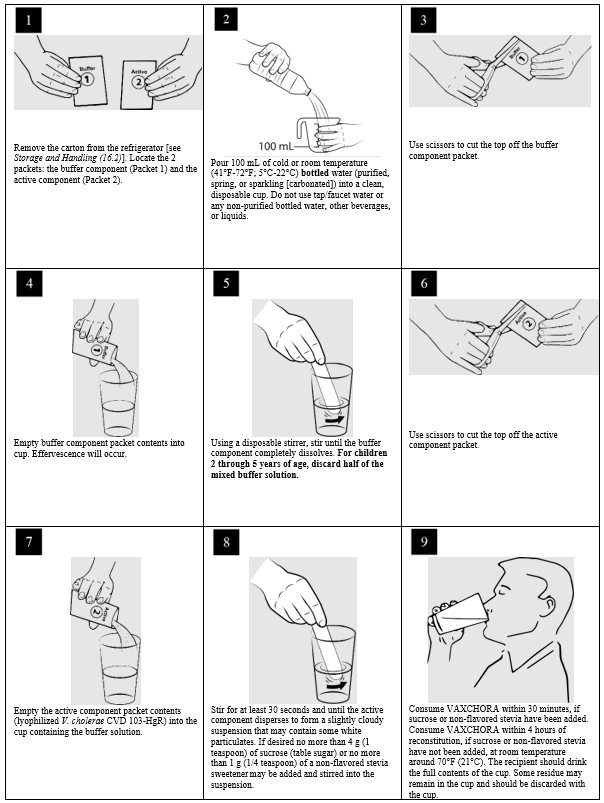
- 1. Remove the carton from the refrigerator [see Storage and Handling (16.2)]. Locate the 2 packets: the buffer component (Packet 1) and the active component (Packet 2).
- 2. Pour 100 milliliters (mL) of cold or room temperature (41°F-72°F; 5°C-22°C) bottled water (purified, spring, or sparkling [carbonated]) into a clean, disposable cup. Do not use tap/faucet water, or any non-purified bottled water, other beverages, or liquids.
- 3. Use scissors to cut the top off the buffer component packet.
- 4. Empty buffer component packet contents into cup. Effervescence will occur.
- 5. Using a disposable stirrer, stir until the buffer component completely dissolves. For children 2 through 5 years of age, discard half of the buffer solution after mixing.
- 6. Use scissors to cut the top off the active component packet.
- 7. Empty the active component packet contents (lyophilized V. cholerae CVD 103-HgR) into the cup containing the buffer solution.
- 8.
Stir for at least 30 seconds and until active component disperses to form a slightly cloudy suspension that may contain some white particulates.
If desired, no more than 4 g (1 teaspoon) of sucrose (table sugar) or no more than 1 g (1/4 teaspoon) of non-flavored stevia sweeteners may be added and stirred into the suspension. Do not add any other sweeteners or medicinal flavorings as this can reduce the effectiveness of the vaccine. - 9.
Consume VAXCHORA within 30 minutes, if sucrose or non-flavored stevia are added. Consume VAXCHORA within 4 hours of reconstitution, if sucrose and non-flavored stevia have not been added, at room temperature around 70 °F (21°C).
The recipient should drink the full contents of the cup. Some residue may remain in the cup and should be discarded with the cup.
NOTE: If the packets are reconstituted in the improper order, the vaccine must be discarded [see Disposal Instructions (2.4)].
-
3 DOSAGE FORMS AND STRENGTHS
VAXCHORA is a suspension for oral administration. Before reconstitution, each dose of VAXCHORA is supplied as a foil packet of buffer and an accompanying foil packet of the active component (lyophilized V. cholerae CVD 103-HgR). After reconstitution, a single dose of VAXCHORA is 100 mL (50 mL for children 2 through 5 years of age).
-
4 CONTRAINDICATIONS
Do not use in persons who have a history of severe allergic reaction (e.g., anaphylaxis) to any ingredient of VAXCHORA or to a previous dose of any cholera vaccine [see Description (11)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Altered Immunocompetence
The safety and effectiveness of VAXCHORA have not been established in immunocompromised persons [see Immunocompromised Individuals (8.6)].
5.2 Shedding and Transmission
VAXCHORA may be shed in the stool of recipients for at least 7 days. There is a potential for transmission of the vaccine strain to non-vaccinated close contacts (e.g., household contacts) [see Pharmacodynamics (12.2)]. Use caution when considering whether to administer VAXCHORA to individuals with immunocompromised close contacts.
-
6 ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a vaccine cannot be directly compared to rates in the clinical trials of another vaccine and may not reflect the rates observed in practice.
The most common adverse reactions for adults (incidence > 3%) were tiredness (31%), headache (29%), abdominal pain (19%), nausea/vomiting (18%), lack of appetite (17%) and diarrhea (4%).
The most common adverse reactions for children and adolescents (incidence ≥10%) were:
- Cohort 1 - age 12 to <18 years: headache (45%), tiredness (41%), abdominal pain (38%), lack of appetite (29%) and nausea (22%).
- Cohort 2 - age 6 to <12 years: tiredness (35%), abdominal pain (27%), headache (26%), lack of appetite (15%) and nausea (14%).
- Cohort 3 - age 2 to <6 years: tiredness (31%), lack of appetite (19%), and abdominal pain (17%).
6.1 Clinical Trials Experience
Trials in Adults
The safety of VAXCHORA was evaluated in four randomized, placebo-controlled, multicenter clinical trials. A total of 3235 adults 18 through 64 years of age received one dose of VAXCHORA and 562 received placebo [physiologic saline (N=551) or lactose (N=11)]. Overall, the mean age was 32.5 years; 53.8% of trial participants were female; 67.1% were White, 27.3% were Black or African American, 1.8% were Asian, 1.7% were multiracial, 1.3% were other, 0.6% were American Indian or Alaskan Native and 0.3% were Native Hawaiian or Pacific Islander. There were 9.3% Hispanic or Latino participants.
Solicited Adverse Reactions
Adults 18 through 45 years of age received VAXCHORA in a multi-center, double-blind, randomized (8:1), placebo-controlled trial conducted in the United States and Australia (Study 1). The safety analysis set included 2789 VAXCHORA recipients. Solicited adverse reactions were recorded daily for 7 days following vaccination. Table 1 presents the frequency and severity of solicited adverse reactions observed within 7 days following receipt of VAXCHORA or placebo in Study 1.
Table 1: Rates of Solicited Adverse Reactions Reported in VAXCHORA Trial Participants 18 to 45 Years of Age During 7 Days Post-Vaccination − Study 1a Adverse Reaction
VAXCHORA
(N=2789)b
%Placebo (Saline)
(N=350)b
%a Data are derived from Study 1 (NCT02094586).
b N represents number of subjects who completed a memory aid.
Grading scales are defined as follows:
Tiredness, Headache, Abdominal Pain, Nausea, Lack of Appetite: Mild = no interference with activity, Moderate = Some interference with activity, Severe = significant, prevents daily activity, Potentially Life Threatening = emergency room (ER) visit or hospitalization.
Vomiting: Mild = 1-2 episodes/24 hours, Moderate = >2 episodes/24 hours, Severe = requires intravenous hydration, Potentially Life Threatening = ER visit or hospitalization for hypotensive shock.
Diarrhea: Mild = 4 loose stools/24 hours, Moderate = 5 loose stools/24 hours, Severe = ≥6 loose stools /24 hours, Potentially Life Threatening = ER visit or hospitalization.
Fever: Mild = 38.0-38.4ºC/100.4-101.1ºF, Moderate = 38.5-38.9ºC/101.2-102.0ºF, Severe = 39.0‑40.0ºC/102.1-104.0ºF, Potentially Life Threatening = >40.0 ºC/104.0 ºF.Tiredness
31.3
27.4
Mild
18.7
16.3
Moderate
12.0
9.9
Severe
0.7
1.2
Potentially life-threatening
0.0
0.0
Headache
28.9
23.6
Mild
18.9
14.6
Moderate
9.6
8.8
Severe
0.5
0.3
Potentially life-threatening
0.0
0.0
Abdominal Pain
18.7
16.9
Mild
12.1
12.0
Moderate
6.2
5.0
Severe
0.4
0.0
Potentially life-threatening
0.0
0.0
Nausea/Vomiting
18.3
15.2
Mild
13.3
11.4
Moderate
4.7
3.8
Severe
0.3
0.0
Potentially life-threatening
0.0
0.0
Lack of Appetite
16.5
16.6
Mild
11.7
12.2
Moderate
4.4
4.4
Severe
0.3
0.0
Potentially life-threatening
0.0
0.0
Diarrhea
3.9
1.2
Mild
2.4
0.9
Moderate
0.7
0.3
Severe
0.8
0.0
Potentially life-threatening
0.04
0.0
Fever
0.6
1.2
Mild
0.2
0.3
Moderate
0.3
0.9
Severe
0.07
0.0
Potentially life-threatening
0.04
0.0
Serious Adverse Events
In a pooled analysis of the four clinical studies, 0.6% (20/3235) of VAXCHORA recipients and 0.5% (3/562) of placebo recipients reported a serious adverse event within 6 months post-vaccination. None of these events were considered to be related to vaccination.
Pediatric Trial
The safety of VAXCHORA in children was evaluated in one randomized, placebo-controlled, multicenter clinical trial. A total of 468 children 2 through 17 years of age received one dose of VAXCHORA and 75 received placebo (physiologic saline). The mean age was 9.0 years; 51.6% were male; 59.5% were White, 31.3% were Black, 7.7% were multiracial, 0.9% were Asian, and 0.6% were American Indian/Alaskan Native. There were 8.7% Hispanic or Latino participants.
Solicited Adverse Reactions
Children 2 through 17 years of age received VAXCHORA in a multi-center, double-blind, randomized (6:1), placebo-controlled trial conducted in the United States (Study 5). Randomization was stratified by age, and children were enrolled in three separate age cohorts: 12 to < 18 years (Cohort 1), 6 to < 12 years (Cohort 2), and 2 to < 6 years (Cohort 3). The safety analysis set included 468 VAXCHORA recipients and 75 placebo recipients. Solicited adverse reactions were recorded daily for 7 days following vaccination. Table 2 presents the frequency and severity of solicited adverse reactions observed within 7 days following receipt of VAXCHORA or placebo in Study 5 for the 3 study cohorts.
Table 2: Rates of Solicited Adverse Reactions Reported in VAXCHORA Pediatric Trial (Study 5) Participants 2 to 17 Years of Age During 7 Days Post-Vaccination by Age cohort Adverse Reaction
Cohort 1
Ages 12 to <18 years VAXCHORA
(N=165)b
%Cohort 1
Ages 12 to <18 years Placebo (Saline)
(N=24)b
%Cohort 2
Ages 6 to <12 years VAXCHORA
(N=157)b
%Cohort 2
Ages 6 to <12 years Placebo (Saline)
(N=25)b
%Cohort 3
Ages 2 to <6 years VAXCHORA
(N=146)b
%Cohort 3
Ages 2 to <6 years Placebo (Saline)
(N=26)b
%a Data are derived from Study 5 (NCT03220737).
b N represents number of subjects with a completed a memory aid.
c Includes 1 case evaluated in the emergency room for an associated viral pharyngitis considered not related to vaccination.
d Includes 1 case with temperature greater than 40°C considered not related to vaccination
Pediatric Grading scales are defined as follows:
Tiredness, Headache, Abdominal Pain, Nausea, Lack of Appetite: Mild = no interference with activity, Moderate = Some interference with activity, Severe = significant, prevents daily activity, Potentially Life Threatening = emergency room (ER) visit or hospitalization.
Vomiting: Mild = 1-2 episodes/24 hours, Moderate = >2 episodes/24 hours, Severe = requires intravenous hydration, Potentially Life Threatening = ER visit or hospitalization for hypotensive shock.
Diarrhea: Mild = 4 loose stools/24 hours, Moderate = 5 loose stools/24 hours, Severe = ≥6 loose stools /24 hours, Potentially Life Threatening = ER visit or hospitalization.
Fever: Mild = 38.0-38.4ºC/100.4-101.1ºF, Moderate = 38.5-38.9ºC/101.2-102.0ºF, Severe = 39.0‑40.0ºC/102.1-104.0ºF, Potentially Life Threatening = >40.0 ºC/104.0 ºF.Tiredness
40.6
37.5
35.0
32.0
30.8
23.1
Mild
31.5
33.3
22.3
20.0
19.2
15.4
Moderate
8.5
0.0
12.1
12.0
11.6
7.7
Severe
0.6 c
4.2
0.6
0.0
0.0
0
Headache
44.8
45.8
26.1
24.0
8.9
7.7
Mild
34.5
45.8
19.1
20.0
6.8
3.8
Moderate
9.7
0.0
5.7
4.0
2.1
3.8
Severe
0.6
0.0
1.3
0.0
0.0
0.0
Abdominal Pain
37.6
16.7
27.4
24.0
17.1
15.4
Mild
28.5
12.5
23.6
16.0
14.4
15.4
Moderate
8.5
4.2
3.8
8.0
2.7
0.0
Severe
0.6
0.0
0.0
0.0
0.0
0.0
Lack of Appetite
29.1
12.5
15.3
20.0
19.2
11.5
Mild
23.6
12.5
12.7
16.0
12.3
7.7
Moderate
5.5
0.0
1.9
4.0
6.8
3.8
Severe
0.0
0.0
0.6
0.0
0.0
0.0
Nausea
22.4
25.0
14.0
16.0
6.8
15.4
Mild
17.0
20.8
12.1
8.0
6.2
15.4
Moderate
4.8
4.2
1.9
8.0
0.7
0.0
Severe
0.6
0.0
0.0
0.0
0.0
0.0
Vomiting
5.5
0.0
4.5
0.0
1.4
11.5
Mild
3.6
0.0
2.5
0.0
1.4
7.7
Moderate
1.2
0.0
1.9
0.0
0.0
3.8
Severe
0.6
0.0
0.0
0.0
0.0
0.0
Fever
1.2
0.0
3.2
4.0
2.1
3.8
Mild
0.6
0.0
0.0
0.0
0.7
0.0
Moderate
0.0
0.0
0.6
4.0
0.7
0.0
Severe
0.6
0.0
2.5
0.0
0.7 d
3.8
Diarrhea
3.6
4.2
0.0
0.0
0.7
0.0
Mild
1.8
0.0
0.0
0.0
0.7
0.0
Moderate
0.0
4.2
0.0
0.0
0.0
0.0
Severe
1.8
0.0
0.0
0.0
0.0
0.0
In total, 13.2% of VAXCHORA recipients reported an unsolicited adverse event that was considered related to study treatment, compared to 9.3% for placebo recipients.
The most frequent unsolicited adverse event for VAXCHORA was loose stool in Cohort 1 (13.9%), Cohort 2 (11.5%) and Cohort 3 (5.5%).
Serious Adverse Events
In Study 5, 0.2% (1/468) of VAXCHORA recipients and 1.3% (1/75) of placebo recipients reported a serious adverse event within 6 months post-vaccination. None of these events were considered to be related to vaccination.
-
7 DRUG INTERACTIONS
7.1 Food and Drink
Avoid food or drink for 60 minutes before and after vaccine administration [see Restrictions on Eating and Drinking (2.2)].
7.2 Concomitant Vaccines or Medications
Vaccines
No data are available on concomitant administration of VAXCHORA with other vaccines.
Antibiotics
Avoid concomitant administration of VAXCHORA with systemic antibiotics since these agents may be active against the vaccine strain and prevent a sufficient degree of multiplication to occur in order to induce a protective immune response. Do not administer VAXCHORA to patients who have received oral or parenteral antibiotics within 14 days prior to vaccination.
Antimalarial Prophylaxis
Data from a study with a similar product indicate that the immune responses to VAXCHORA may be diminished when VAXCHORA is administered concomitantly with chloroquine. Administer VAXCHORA at least 10 days before beginning antimalarial prophylaxis with chloroquine.
7.3 Immunosuppressive Treatments
Immunosuppressive therapies, including irradiation, antimetabolites, alkylating agents, cytotoxic drugs and corticosteroids (used in greater than physiologic doses), may reduce the immune response to VAXCHORA [see Use in Specific Populations (8.6)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
VAXCHORA is not absorbed systemically following oral administration, and maternal use is not expected to result in fetal exposure to the drug.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Maternal cholera disease is associated with adverse pregnancy outcomes including fetal death.
Fetal/neonatal adverse reactions
The vaccine strain may be shed in the stool of the vaccinated mother for at least 7 days, with a potential for transmission of the vaccine strain from mother to infant during vaginal delivery.
8.2 Lactation
Risk Summary
VAXCHORA is not absorbed systemically by the mother following oral administration, and breastfeeding is not expected to result in exposure of the child to VAXCHORA.
8.4 Pediatric Use
The safety and effectiveness of VAXCHORA have not been established in children younger than 2 years of age.
8.5 Geriatric Use
The safety and effectiveness of VAXCHORA have not been established in adults 65 years of age or older.
8.6 Immunocompromised Individuals
The safety and effectiveness of VAXCHORA have not been established in immunocompromised individuals. The immunologic response to VAXCHORA may be diminished in immunocompromised individuals [see Drug Interactions (7.3)].
-
11 DESCRIPTION
VAXCHORA (Cholera Vaccine, Live, Oral) is a live, attenuated bacterial vaccine suspension for oral administration containing the V. cholerae strain CVD 103-HgR. CVD 103-HgR was constructed from the serogroup O1 classical Inaba strain 569B by deleting the catalytic domain sequence of both copies of the ctxA gene, which prevents the synthesis of active cholera toxin (CT). This attenuated strain remains able to synthesize the immunogenic non-toxic B subunit of CT (encoded by the ctxB gene). In addition, a marker was inserted into the hemolysin gene locus (hlyA) to enable differentiation of the vaccine strain from wild type V. cholerae O1.
The vaccine strain is grown in fermentors under controlled conditions in medium containing casamino acids, yeast extract, mineral salts, and an anti-foaming agent. The bacteria are concentrated by ultrafiltration before addition of a stabilization solution containing ascorbic acid (an antioxidant), Hy-Case SF (hydrolyzed casein [a protein derived from cow's milk], a cryoprotectant), and sucrose (a cryoprotectant). The stabilized bacteria are lyophilized, milled, and blended with anhydrous lactose (a bulking agent). The active component blend is filled into packets.
The buffer component is manufactured by blending together sodium bicarbonate (a gastric acid neutralizer), sodium carbonate (a buffer), ascorbic acid (a buffer and water chlorine neutralizer), and anhydrous lactose (a manufacturing flow aid). The buffer component blend is filled into packets. One buffer component packet and one active component packet are packaged into individual single dose cartons for distribution.
After reconstitution, VAXCHORA contains 4 x 108 to 2 x 109 colony forming units (CFU) of live attenuated V. cholerae CVD 103-HgR. The resulting suspension should be slightly cloudy and may contain white particulates. The active and buffer ingredients are shown in Table 3.
Table 3: Vaccine Composition a CFU=colony forming units.
b mg=milligrams.
c g=grams.Active Component Packet
Ingredient
Active Component Packet
Quantity/packet
V. cholerae CVD 103-HgR
4 x 108 to 2 x 109 CFUa
Sucrose
≤ 165.37 mgb
Hy-Case SF (hydrolyzed casein)
≤ 17.11 mg
Ascorbic acid
≤ 8.55 mg
Anhydrous lactose
≤ 2.09 gc
Buffer Component Packet
Ingredient
Buffer Component Packet
Quantity/packet
Sodium bicarbonate
2.16–2.41 g
Sodium carbonate
0.24-0.49 g
Ascorbic acid
1.50–1.80 g
Anhydrous lactose
0.18–0.22 g
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
VAXCHORA contains live attenuated cholera bacteria that replicate in the gastrointestinal tract of the recipient. Immune mechanisms conferring protection against cholera following receipt of VAXCHORA have not been determined. However, rises in serum vibriocidal antibody 10 days after vaccination with VAXCHORA were associated with protection in a human challenge study (Study 2) [see Immunogenicity (14.2)].
12.2 Pharmacodynamics
Shedding of the vaccine strain was evaluated in the first 7 days post-vaccination in a study of 53 healthy adult vaccine recipients (Study 3). VAXCHORA was shed in the stools of 11.3% [95% CI 4.3%, 23.0%] of vaccine recipients on any day through 7 days post-vaccination. During the 7 days post-vaccination, the proportion of subjects shedding was highest on day 7 (7.5% [95% CI 2.1%, 18.2%]). The duration of shedding of the vaccine strain is unknown.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
14.1 Efficacy Against V. cholerae Challenge
Study 2 was a randomized, double-blind, saline placebo-controlled V. cholerae challenge study conducted in the US. Subjects 18 through 45 years of age (N=197) with no prior history of cholera infection or travel to a cholera-endemic area in the previous 5 years were randomized according to a 1:1 ratio to receive one dose of VAXCHORA or placebo. In order to identify the subset of subjects to be challenged, an unblinded statistician prepared four randomly ordered lists of subjects per site, one list each for vaccine recipients with blood type O, vaccine recipients with non-O blood types, placebo recipients with blood type O, and placebo recipients with non-O blood types. This was done to maintain a minimum of 60% blood group O subjects in each treatment group. Individuals with type O blood are less likely to be infected with V. cholerae, but are at risk for developing severe cholera if infected. Each site was provided with a blinded version of the four lists specific to its site and advised on the number of subjects from each list to challenge. In the event that a subject was determined to be ineligible for challenge, the site was instructed to select the next subject from the same list as the ineligible subject.
The challenges were split into 2 cohorts for 10 day and 3 month challenges. Subjects were admitted to an inpatient unit. Subjects had nothing by mouth from midnight before ingestion of the challenge strain, except for water, and had nothing by mouth for 90 minutes after ingestion of the challenge strain. Approximately 1 minute prior to challenge, subjects ingested 120 mL sodium bicarbonate (NaHCO3) buffer. The oral challenge consisted of 1 x 105 CFU live wild type V. cholerae El Tor Inaba N16961 in 30 mL NaHCO3 buffer at 10 days or 3 months post-vaccination. The co-primary objectives were to demonstrate the efficacy of a single dose of VAXCHORA in the prevention of moderate to severe diarrhea following challenge at 10 days and 3 months post-vaccination. Moderate to severe diarrhea was defined as cumulative diarrheal purge ≥ 3 liters (L) within 10 days after challenge. Diarrheal stool was defined as ≥ 2 unformed stools (takes shape of container) collected during a 48 hour period ≥ 200 grams (g) or a single unformed stool ≥ 300 g. Subjects were instructed to collect every stool from the time of challenge until discharge from the inpatient unit. Nursing staff or study personnel inspected all stool, graded the consistency of the stool and calculated the total weight of diarrheal stool per day. Weight of stool was converted to volume using the formula 1 g=1 mL. VAXCHORA recipients challenged at 10 days post-vaccination and VAXCHORA recipients challenged at 3 months post-vaccination were compared with a pooled group of placebo (saline) recipients challenged at 10 days or 3 months post-vaccination.
Of the 95 VAXCHORA recipients, 68 were challenged; 35 were challenged at 10 days post-vaccination and 33 were challenged at 3 months post-vaccination. Of the 102 placebo recipients, 66 were challenged; 33 were challenged at 10 days post-vaccination and 33 at 3 months post-vaccination. Among all randomized subjects, the mean age was 31.0 years. Overall, the mean age of the challenge population was 31.4 years. More males were in the vaccine group (71.6%) compared to the placebo group (54.9%). The majority of randomized subjects were Black (67.5%), 29.4% were White, 0.5% were American Indian/Alaskan Native, 0.5% were Asian, and 2.0% were other. There were 4.6% Hispanic or Latino participants. Overall 50.3% had blood type O. Among subjects selected for either challenge cohort, more males were challenged in the vaccine group (76.5%) compared to the placebo group (57.6%). The majority (70.9%) of the challenge population were Black, 25.4% were White, 0.7% were American Indian/Alaskan Native, 0.7% were Asian, and 2.2% were other. There were 3.7% Hispanic or Latino participants. Overall, 56.0% of challenged subjects had blood type O.
Vaccine efficacy against the occurrence of moderate to severe diarrhea at 10 days post-vaccination was 90.3% [95% CI 62.7%, 100.0%] and at 3 months post-vaccination was 79.5% [95% CI 49.9%, 100.0%] (Table 4).
Table 4: Vaccine Efficacy in the Prevention of Moderate to Severe Diarrhea Following Challenge with V. cholerae O1 El Tor Inaba at 10 Days and 3 Months Post-Vaccination (Intent-to-Treat Population) Parameter VAXCHORA
10 Day Challengea,c
N=35dVAXCHORA
3 Month Challengea,c
N=33dCombined Placeboa,b
10 Day or 3 Month Challengec
N=66da Data are derived from Study 2 (NCT01895855).
b Combined placebo group comprised of all placebo recipients who were challenged at either 10 days (N=33) or 3 months (N=33) following vaccination.
c Challenge strain was V. cholerae O1 El Tor Inaba N16961.
d N=number of subjects challenged in each group.
e Moderate or severe diarrhea (≥ 3 liters of diarrhea) within 10 days after challenge.
f Vaccine Efficacy=[(Attack Rate in Placebo Group - Attack Rate in Vaccine Group)/Attack Rate in Placebo Group] x 100.
g Pre-specified criteria for success were that the lower bound of the two-sided 95% confidence interval for vaccine efficacy must be ≥30% in both the 10 Day and 3 Month challenge groups.
h CI=confidence interval.Number of Subjects with Moderate or Severe Diarrhea (Attack Rate)e
2 (5.7%)
4 (12.1%)
39 (59.1%)
Vaccine Efficacy %f,g
[95% CIh]90.3%
[62.7%, 100.0%]79.5%
[49.9%, 100.0%]14.2 Immunogenicity
Vibriocidal Antibody Against the Vaccine Strain (classical Inaba)
A vibriocidal antibody assay was used to measure serum levels of neutralizing antibodies against the vaccine strain.
Study 2 was a randomized, double-blind, saline placebo-controlled V. cholerae challenge study conducted in adults 18 through 45 years of age. In the subset of subjects challenged in Study 2, 91% [95% CI 82%, 97%] of vaccinees seroconverted prior to challenge and 9% developed moderate to severe cholera following challenge, while 2% of placebo recipients seroconverted prior to challenge and 59% developed moderate to severe cholera following challenge. Seroconversion was defined as a ≥ 4-fold rise in serum vibriocidal antibody from baseline to 10 days post vaccination. Based on the observed association between seroconversion and protection from V. cholerae disease, seroconversion rate at 10 days post-vaccination was used to evaluate response to vaccination in other age groups.
Study 1 was a randomized, double-blind, saline placebo-controlled safety and immunogenicity study conducted in the US and Australia. A total of 3146 subjects 18 through 45 years of age not previously exposed to cholera were randomized 8:1 to receive one dose of VAXCHORA or placebo. The mean age was 29.9 years; 45.2% were male; 68.3% were White, 25.6% were Black, 2.0% were Asian, 1.9% were multiracial, 1.4% were other, 0.4% were American Indian/Alaskan Native, and 0.3% were Native Hawaiian/Pacific Islander. There were 10.0% Hispanic or Latino participants.
In this study, classical Inaba vibriocidal antibody seroconversion rates were 93.5% [95% CI 92.5%, 94.4%] in vaccine recipients and 4% [95% CI 2%, 7%] in placebo recipients at 10 days post-vaccination.
Study 4 was a randomized, double-blind, placebo-controlled safety and immunogenicity study conducted in the US. A total of 398 subjects 46 through 64 years of age with no prior history of cholera infection or travel to a cholera-endemic area in the previous 5 years were randomized 3:1 to receive one dose of VAXCHORA or placebo. Overall, the mean age of the randomized population was 53.8 years; 45.7% were male; 74.9% were White, 21.9% were Black, 1.8% were American Indian/Alaskan Native, 0.5% were Asian, 0.5% were multiracial, 0.3% were Native Hawaiian/Pacific Islander, and 0.3% were other. There were 7.5% Hispanic or Latino participants.
Vibriocidal antibody seroconversion rates at 10 days post-vaccination for the classical Inaba strain among 46 through 64-year-old subjects in Study 4 were compared to those in 18 through 45-year-old subjects in Study 1. VAXCHORA recipients from Study 1 were in the same age group as those in Study 2, the V. cholerae challenge study.
Adults 46 through 64 years were shown to have a non-inferior rate of classical Inaba vibriocidal antibody seroconversion at 10 days post-vaccination compared to adults 18 through 45 years of age (Table 5).
Table 5: Vibriocidal Antibody Seroconversion Against Classical Inaba V. cholerae Vaccine Strain at 10 Days Post-Vaccination in Adults 46 through 64 Years of Age (Study 4) Compared to Adults 18 through 45 Years of Age (Study 1) [Bridging Analysis Population] Study a Dose/CFU VAXCHORA
NbVAXCHORA
Seroconversion
%
[95% CIc]a Data are derived from Study 1 (NCT02094586) and Study 4 (NCT02100631).
b N=number of subjects with analyzable samples at Day 1 and Day 11.
c CI=confidence interval.
d Seroconversion is defined as the percentages of subjects who had at least a 4-fold rise in vibriocidal antibody titer at 10 days post-vaccination compared to baseline.
e Pre-specified success criterion was that the lower bound of the two-sided 95% confidence interval on the difference in seroconversion rate (Study 4 minus Study 1) must be greater than -10 percentage points.Study 4
(46 through 64-year-olds)1 x 109
291
90.4%
[86.4%, 93.5%]Study 1
(18 through 45-year-olds)1 x 109
2687
93.5%
[92.5%, 94.4%]Difference in Seroconversion Ratesd,e
−3.1%
[−6.7%, 0.4%]Vibriocidal Antibody Against Classical Ogawa, El Tor Inaba and El Tor Ogawa
V. cholerae serogroup O1 consists of four major subtypes: classical Inaba, classical Ogawa, El Tor Inaba and El Tor Ogawa. Serum vibriocidal antibody against the three types of V. cholerae not contained in the vaccine, namely classical Ogawa, El Tor Inaba and El Tor Ogawa, was also measured in Study 2 and Study 4. The percentages of vaccine recipients who seroconverted against each of the 4 major biotype/serotypes of V. cholerae serogroup O1 at 10 days post-vaccination (71.4% to 91.4%) are shown in Table 6.
Table 6: Seroconversion Rates 10 Days Post-Vaccination for the Four Major V. cholerae O1 Serogroup Biotypes and Serotypes in Studies 2 and 4 [Immunogenicity Evaluable Population] Cholera Strain Study 2a
(18 through 45-year-olds)
VAXCHORA
NbStudy 2a
(18 through 45-year-olds)
VAXCHORA
%c
[95% CId]Study 4a
(46 through 64-year-olds)
VAXCHORA
NbStudy 4a
(46 through 64-year-olds)
VAXCHORA
%
[95% CI]a Data are derived from Study 2 (NCT01895855) and Study 4 (NCT02100631).
b N=number of subjects with measurements at baseline and 10 days post-vaccination. One subject in Study 2 did not have a Day 11 measurement and was dropped from the analysis.
c Seroconversion is defined as the percentages of subjects who had at least a 4-fold rise in vibriocidal antibody titer at 10 days post-vaccination compared to the titer measured at baseline.
d CI=confidence interval.
e VAXCHORA contains the classical Inaba strain of V. cholerae O1.Classical Inabae
93
90.3%
[82.4%, 95.5%]291
90.4%
[86.4%, 93.5%]El Tor Inaba
93
91.4%
[83.8%, 96.2%]290
91.0%
[87.1%, 94.1%]Classical Ogawa
93
87.1%
[78.5%, 93.2%]291
73.2%
[67.7%, 78.2%]El Tor Ogawa
93
89.2%
[81.1%, 94.7%]290
71.4%
[65.8%, 76.5%]Pediatric Trial - Vibriocidal Antibody Against the Vaccine Strain (classical Inaba)
The effectiveness of VAXCHORA for the pediatric population 2 through 17 years of age was demonstrated following comparison of the immune response to V. cholerae in children and adolescents to the immune response in adults following Vaxchora (immunobridging).
Study 5 was a randomized, double-blind, saline placebo-controlled safety and immunogenicity study conducted in the US. A total of 550 subjects 2 through 17 years of age not previously exposed to cholera were randomized 6:1 to receive one dose of VAXCHORA (1 x 109 CFU/dose) or placebo. Randomization was stratified by age into 3 age cohorts:
- Cohort 1: 12 to <18 years of age
- Cohort 2: 6 to <12 years of age
- Cohort 3: 2 to <6 years of age
In this study, classical Inaba vibriocidal antibody seroconversion rates at 10-days post-vaccination in children 2 through 17 years of age were compared to seroconversion rates in adults 18 through 45 years of age.
Immunobridging results for VAXCHORA, at 10 days post-vaccination, are shown in Table 7. The rate of seroconversion among pediatric placebo recipients was 1.5% [95% CI 0.3%, 8.0%] at 10 days post-vaccination.
Table 7: Vibriocidal Antibody Seroconversion Against Classical Inaba V. cholerae Vaccine Strain at 10 Days Post-Vaccination in Children aged 2 through 17 Years (Study 5) Compared to Adults 18 through 45 Years of Age (Study 1) [Bridging Analysis Populationb] Study a Dose/CFU VAXCHORA
NbVAXCHORA
Seroconversion
%
[98.3% CIc]a Data are derived from Study 1 (NCT02094586) and Study 5 (NCT03220737).
b N=number of subjects with analyzable samples at Day 1 and Day 11 in the immunogenicity evaluable population.
c CI=confidence interval.
d Seroconversion is defined as the percentages of subjects who had at least a 4-fold rise in vibriocidal antibody titer at 10 days post-vaccination compared to baseline.
e Pre-specified success criterion was that the lower bound of the two-sided 96.7% confidence interval on the difference in seroconversion rate (Study 5 minus Study 1) must be greater than -10 percentage points. Co-primary criterion required the lower limit of the 98.3% CI to be ≥ 70% for the VAXCHORA group.Study 5
(2 through 17-year-olds)1 x 109
399
98.5%
[96.2%, 99.4%]Study 1
(18 through 45-year-olds)1 x 109
2687
93.5%
[92.3%, 94.6%]Difference in Seroconversion Ratesd,e
5.0%
[2.8%, 6.4%] -
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
VAXCHORA is supplied as shown in Table 8. The contents of both packets are reconstituted with bottled water (purified, spring, or sparkling [carbonated]), to form one oral dose of the vaccine.
Table 8: VAXCHORA Product Presentation Presentation Carton NDC Number Components Single dose carton containing two packets
NDC: 50632-015-02
Buffer Component Packet NDC: 50632-015-02
Active Component Packet NDC: 50632-015-02 -
17 PATIENT COUNSELING INFORMATION
Prior to administration of this vaccine, the health care professional should inform the individual of the following:
- Advise vaccine recipients to exercise caution regarding food and water consumed in cholera-affected areas, in accordance with the recommendations from the Centers for Disease Control and Prevention for the prevention of cholera in travelers.
- Educate vaccine recipients regarding the most common adverse reactions occurring within 7 days post-vaccination with VAXCHORA (tiredness, headache, abdominal pain, nausea/vomiting, lack of appetite, and diarrhea).
- Inform vaccine recipients that VAXCHORA is a live attenuated vaccine and has the potential for transmission of the vaccine strain to close contacts (e.g., household contacts). For at least 14 days following vaccination with VAXCHORA, vaccine recipients should wash their hands thoroughly after using the bathroom and before preparing or handling food.
- Instruct vaccine recipients to report adverse reactions to their healthcare provider.
VAXCHORA® is a registered trademark of Bavarian Nordic A/S
US License No. 2096
Manufactured by: Bavarian Nordic A/S, Philip Heymans Alle 3, 2900 Hellerup, Denmark
© 2023 Bavarian Nordic A/S. All rights reserved.
-
PRINCIPAL DISPLAY PANEL - Carton
NDC: 50632-015-02
STORE REFRIGERATED
Use After ReconstitutionCholera Vaccine, Live, Oral
Vaxchora®Contents: Single-dose Active Packet (4x108 to 2x109 CFU of
Vibrio cholerae CVD 103-HgR) and Single-dose Buffer Packet.Prior to administration, reconstitute Buffer in 100 mL of
bottled water (purified, spring or sparkling). For children
under 6 years of age only, discard half the buffer solution.
After reconstitution, then add Active.
See package insert.Cholera Vaccine, Live, Oral
Vaxchora®Bavarian Nordic A/S, Denmark
BAVARIAN NORDICCholera Vaccine, Live Oral
Vaxchora®Live vaccine for oral, active immunization against cholera
Store at 36 °F to 46 °F (2 °C to 8 °C)Administration and dosage: see package insert
Manufactured by:
Bavarian Nordic A/S
Philip Heymans Alle 3
2900 Hellerup, DenmarkUS License No. 2096
Rx ONLY
GTIN:
S/N:
EXP:
LOT: -
PRINCIPAL DISPLAY PANEL - Buffer and Active Packets
BUFFER COMPONENT
OF VAXCHORA®
(Cholera Vaccine, Live, Oral)1
Packet 1 of 2. USE FIRST.
Contents (Single-Dose): Buffer
Directions: Add entire contents
of Packet 1 to 100 mL of bottled
water (purified, spring or sparkling)
in disposable cup, then stir.
For children under 6 years of age
only, discard half the buffer solution.
Go to Packet 2. See package insert.Rx Only - For Oral Administration
EXP: LOT:
NDC: 50632-015-02
Bavarian Nordic A/S
US License No. 2096ACTIVE COMPONENT
of VAXCHORA®
(Cholera Vaccine, Live, Oral)2
Packet 2 of 2. USE LAST.
Contents (Single-Dose):
4x108 to 2x109 CFU of
lyophilized Vibrio cholerae
CVD 103-HgR.Directions: Add contents of Packet 2
to cup and stir to create vaccine. See
package insert.Rx Only - For Oral Administration
EXP: LOT:
NDC: 50632-015-02
Bavarian Nordic A/S
US License No. 2096 -
INGREDIENTS AND APPEARANCE
VAXCHORA
cholera vaccine, live, oral kitProduct Information Product Type VACCINE Item Code (Source) NDC: 50632-015 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50632-015-02 1 in 1 CARTON; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 PACKET 1 Part 2 1 PACKET 1 Part 1 of 2 VAXCHORA
cholera vaccine, live, oral powder, for suspensionProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VIBRIO CHOLERAE CVD 103-HGR STRAIN LIVE ANTIGEN (UNII: V9G528E9E0) (VIBRIO CHOLERAE CVD 103-HGR STRAIN LIVE ANTIGEN - UNII:V9G528E9E0) VIBRIO CHOLERAE CVD 103-HGR STRAIN LIVE ANTIGEN 1200000000 [CFU] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) HYDROLYZED CASEIN (ENZYMATIC; 1000 MW) (UNII: M93H91U80R) ASCORBIC ACID (UNII: PQ6CK8PD0R) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125597 06/01/2024 Part 2 of 2 BUFFER
buffer powder, for suspensionProduct Information Route of Administration ORAL Inactive Ingredients Ingredient Name Strength SODIUM BICARBONATE (UNII: 8MDF5V39QO) SODIUM CARBONATE (UNII: 45P3261C7T) ASCORBIC ACID (UNII: PQ6CK8PD0R) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125597 06/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125597 06/01/2024 Labeler - Bavarian Nordic A/S (310209754) Establishment Name Address ID/FEI Business Operations Bavarian Nordic Berna GmbH 480030654 ANALYSIS(50632-015) , LABEL(50632-015) , MANUFACTURE(50632-015) , PACK(50632-015)
Trademark Results [Vaxchora]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VAXCHORA 86981735 5219638 Live/Registered |
EMERGENT TRAVEL HEALTH INC. 2014-02-26 |
 VAXCHORA 86205531 not registered Dead/Abandoned |
PAXVAX BERMUDA LTD. 2014-02-26 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.