MIGRAINE PAIN MANAGEMENT- iris versicolor root and sodium chloride solution/ drops
Migraine by
Drug Labeling and Warnings
Migraine by is a Homeopathic medication manufactured, distributed, or labeled by Forces of Nature. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Also Contains
- Indications
- Directions
-
Warnings
Some individuals may be sensitive to essential oils. Begin with a small drop to determine if the product causes any increased redness or irritation and discontinue use if it irritates your skin.
For external use only. If accidental ingestion of more than several drops, and adverse reaction occurs, get medical help or contact a Poison Control Center. Do not apply to the eyes. If you are pregnant or breast - feeding, ask a health professional before use. Not for use on children under the age of 3.
- SPL UNCLASSIFIED SECTION
-
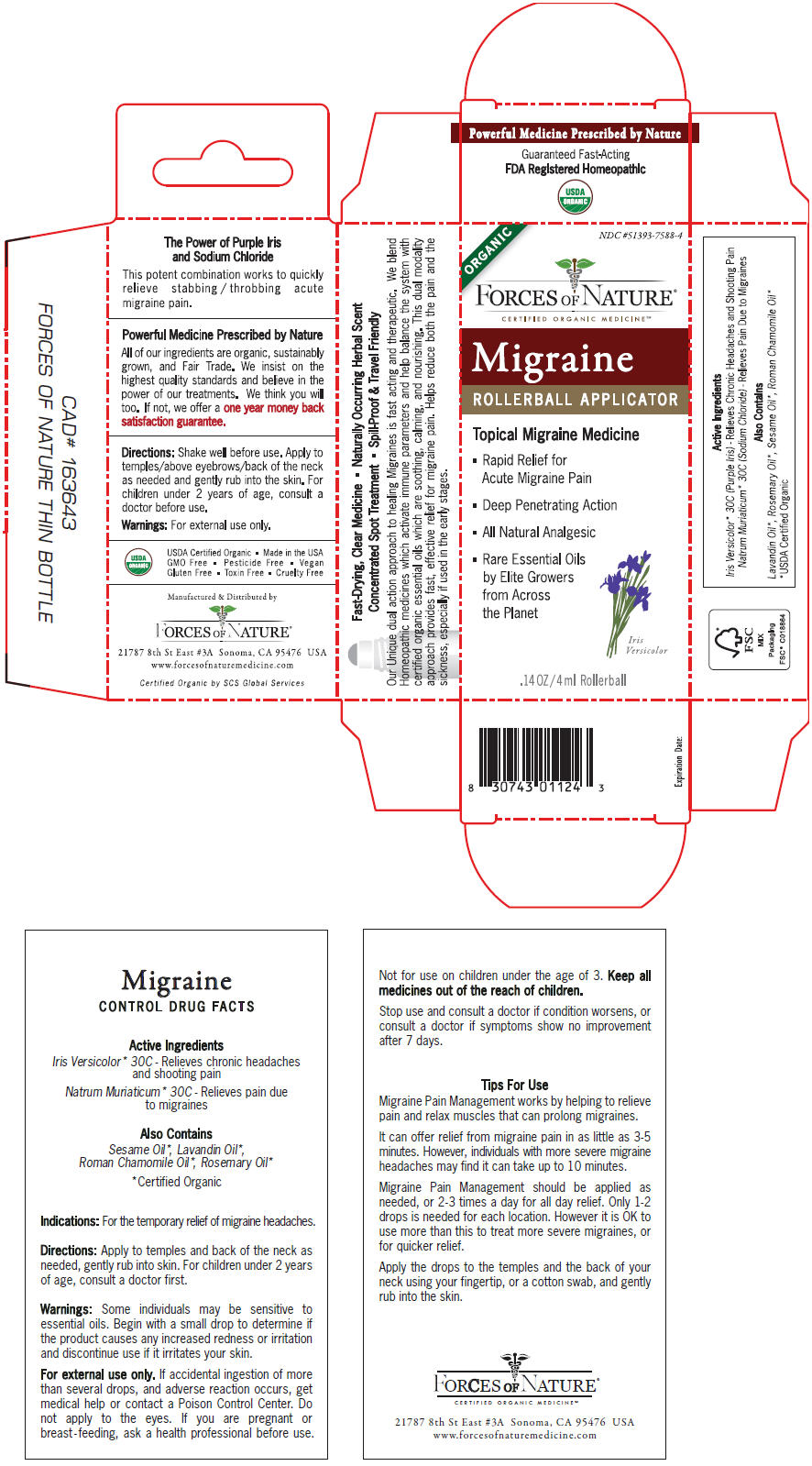
PRINCIPAL DISPLAY PANEL - 4 ml Bottle Carton
ORGANIC
NDC #51393-7588-4
FORCES OF NATURE®
CERTIFIED ORGANIC MEDICINESMMigraine
ROLLERBALL APPLICATORTopical Migraine Medicine
- Rapid Relief for
Acute Migraine Pain - Deep Penetrating Action
- All Natural Analgesic
- Rare Essential Oils
by Elite Growers
from Across
the Planet
Iris
Versicolor.14 OZ/4 ml Rollerball
- Rapid Relief for
-
INGREDIENTS AND APPEARANCE
MIGRAINE PAIN MANAGEMENT
iris versicolor root and sodium chloride solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 51393-7588 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Iris Versicolor Root (UNII: X43D4L3DQC) (Iris Versicolor Root - UNII:X43D4L3DQC) Iris Versicolor Root 30 [hp_X] in 100 mL Sodium Chloride (UNII: 451W47IQ8X) (Sodium Cation - UNII:LYR4M0NH37) Sodium Chloride 30 [hp_C] in 100 mL Inactive Ingredients Ingredient Name Strength Chamaemelum Nobile Flower Oil (UNII: UB27587839) Lavandin Oil (UNII: 9RES347CKG) Rosemary Oil (UNII: 8LGU7VM393) Sesame Oil (UNII: QX10HYY4QV) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51393-7588-1 11 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 12/01/2011 2 NDC: 51393-7588-2 33 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 12/01/2011 3 NDC: 51393-7588-4 4 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 07/01/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED HOMEOPATHIC 12/01/2011 Labeler - Forces of Nature (050169130)
Trademark Results [Migraine]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MIGRAINE 88341211 not registered Live/Pending |
J&E United, LLC 2019-03-15 |
 MIGRAINE 88007204 not registered Live/Pending |
J&E United, LLC 2018-06-20 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.