WILEY SPINAL 199-2340- kit
Wiley Spinal by
Drug Labeling and Warnings
Wiley Spinal by is a Other medication manufactured, distributed, or labeled by Epimed International, Aplicare, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
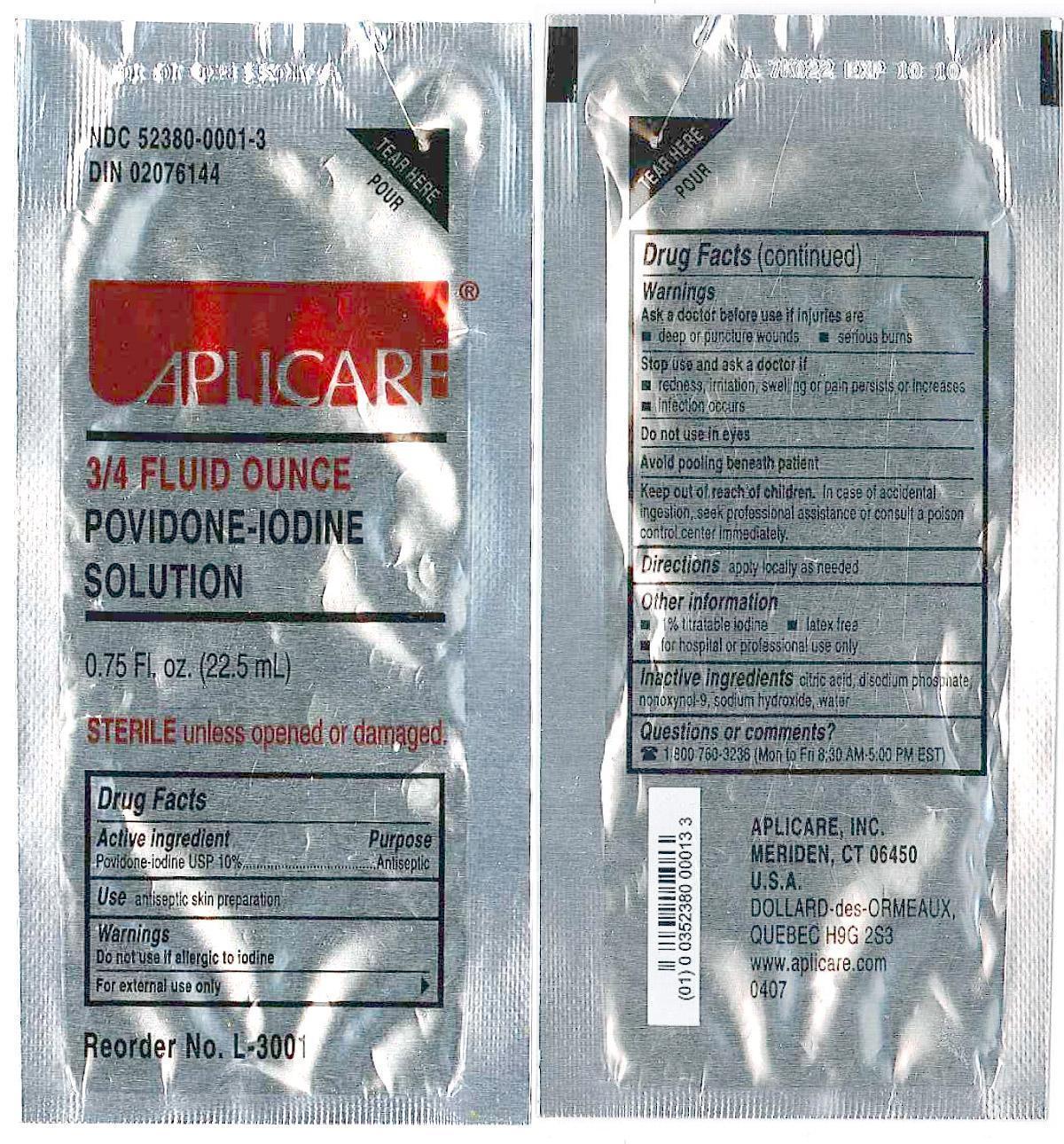
- DESCRIPTION
-
WARNINGS
Povidone-iodine 10%
Antiseptic
Warnings
Do not use
- if allergic to iodine
- in the eyes
For external use only
Ask a doctor before use if injuries are
- deep or puncture wounds
- serious burns
Stop use and ask a doctor if
- redness, irritation, swelling or pain persists or increases
- infection occurs
Avoid pooling beneath patient
Keep out of reach of children.In case of accidental ingestion, seek professionalassistance or consult a poison control center immediately.
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
WILEY SPINAL 199-2340
needle, conduction, anesthetic (w/wo introducer) kitProduct Information Product Type MEDICAL DEVICE Item Code (Source) NHRIC:60816-2340 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:60816-2340-1 1 in 1 PACKAGE Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 PACKET 22.5 mL Part 1 of 1 APLICARE POVIDONE-IODINE
povidone-iodine solutionProduct Information Item Code (Source) NDC: 52380-0001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Povidone-iodine (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) SODIUM HYDROXIDE (UNII: 55X04QC32I) NONOXYNOL-9 (UNII: 48Q180SH9T) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 22.5 mL in 1 PACKET Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date premarket notification K043467 10/08/1996 Labeler - Epimed International (799751953) Establishment Name Address ID/FEI Business Operations Epimed International 799751953 relabel, manufacture Establishment Name Address ID/FEI Business Operations Aplicare, Inc. 107255002 manufacture
Trademark Results [Wiley Spinal]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 WILEY SPINAL 85313663 4181831 Live/Registered |
CUSTOM MEDICAL APPLICATIONS 2011-05-05 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.