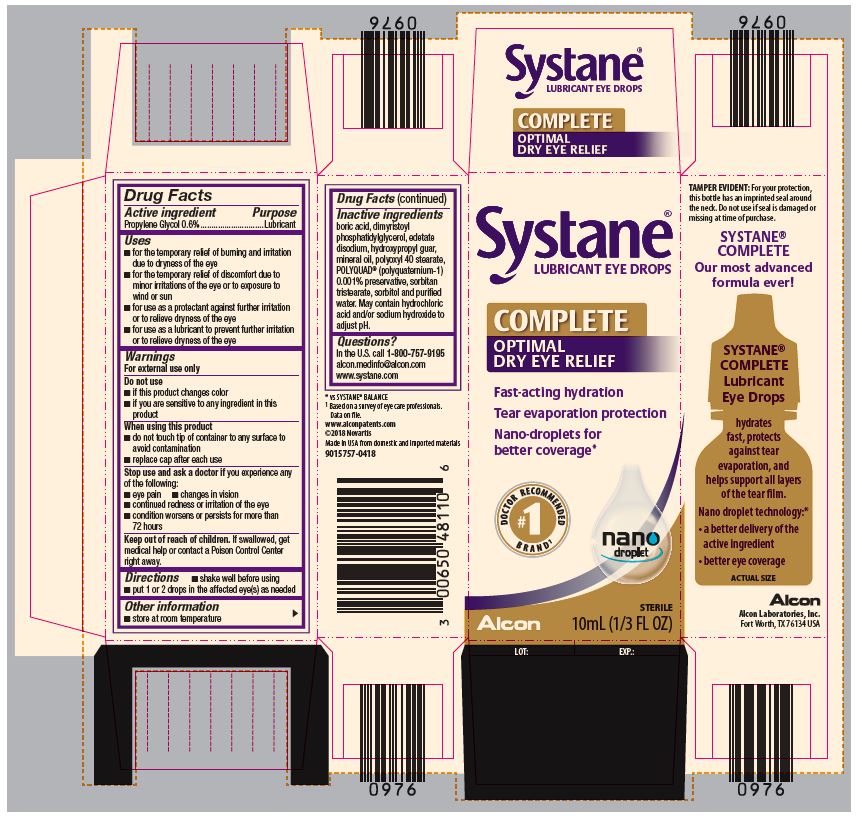
SYSTANE COMPLETE- propylene glycol solution/ drops
Systane COMPLETE by
Drug Labeling and Warnings
Systane COMPLETE by is a Otc medication manufactured, distributed, or labeled by Alcon Laboratories, Inc., Alcon Research LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
-
Uses
- for the temporary relief of burning and irritation due to dryness of the eye
- for the temporary relief of discomfort due to minor irritations of the eye or to exposure to wind or sun
- for use as a protectant against further irritation or to relieve dryness of the eye
- for use as a lubricant to prevent further irritation or to relieve dryness of the eye
-
Warnings
For external use only
When using this product
- do not touch tip of container to any surface to avoid contamination
- replace cap after each use
- Directions
- Other Information
- Inactive Ingredients
- Questions?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SYSTANE COMPLETE
propylene glycol solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0065-0481 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Propylene Glycol (UNII: 6DC9Q167V3) (Propylene Glycol - UNII:6DC9Q167V3) Propylene Glycol .06 mg in 1 mL Inactive Ingredients Ingredient Name Strength Boric Acid (UNII: R57ZHV85D4) Dimyristoylphosphatidylglycerol, Dl- (UNII: BI71WT9P3R) Edetate Disodium (UNII: 7FLD91C86K) Guar Gum (UNII: E89I1637KE) Mineral Oil (UNII: T5L8T28FGP) Polyoxyl 40 Stearate (UNII: 13A4J4NH9I) Sorbitan Tristearate (UNII: 6LUM696811) Sorbitol (UNII: 506T60A25R) Water (UNII: 059QF0KO0R) Hydrochloric Acid (UNII: QTT17582CB) Sodium Hydroxide (UNII: 55X04QC32I) Polidronium Chloride (UNII: 6716Z5YR3G) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0065-0481-10 1 in 1 CARTON 01/18/2018 1 10 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 2 NDC: 0065-0481-11 2 in 1 CARTON 01/18/2018 2 10 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 3 NDC: 0065-0481-01 1 in 1 CARTON 04/23/2018 3 1.5 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 4 NDC: 0065-0481-55 1 in 1 CARTON 12/10/2018 4 5 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 5 NDC: 0065-0481-72 3 in 1 CARTON 02/14/2020 5 10 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part349 01/18/2018 Labeler - Alcon Laboratories, Inc. (008018525) Establishment Name Address ID/FEI Business Operations Alcon Research LLC 007672236 manufacture(0065-0481)
Trademark Results [Systane COMPLETE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SYSTANE COMPLETE 87544855 not registered Dead/Abandoned |
Novartis AG 2017-07-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.