CLINDACARE- clindamycin, ibuprofen, chlorhexidine gluconate kit
ClindaCare by
Drug Labeling and Warnings
ClindaCare by is a Prescription medication manufactured, distributed, or labeled by Brisk Pharmaceuticals. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
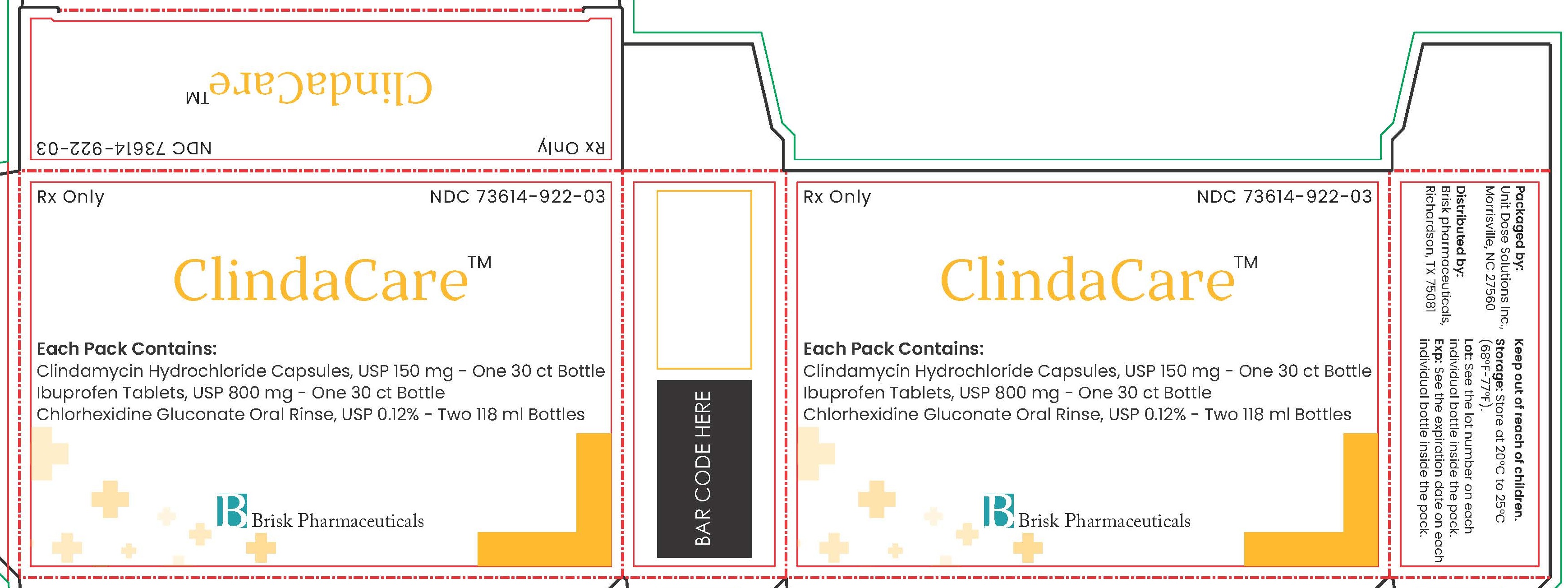
Rx Only
NDC: 73614-922-03
ClindaCare™
Each Pack Contains:
Clindamycin Hydrochloride Capsules, USP 150 mg - One 30 ct Bottle
Ibuprofen Tablets, USP 800 mg - One 30 ct Bottle
Chlorhexidine Gluconate Oral Rinse, USP 0.12% - Two 118 ml Bottles
Brisk Pharmaceuticals
-
DESCRIPTION
Packaged by:
Unit Dose Solutions Inc.,
Morrisville, NC 27560
Distributed by:
Brisk pharmaceuticals,
Richardson, TX 75081
Keep out of reach of children.
Storage: Store at 20°C to 25°C
(68°F-77°F).
Lot: See the lot number on each
individual bottle inside the pack.
Exp: See the expiration date on each
individual bottle inside the pack.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CLINDACARE
clindamycin, ibuprofen, chlorhexidine gluconate kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 73614-922 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73614-922-03 1 in 1 CARTON; Type 1: Convenience Kit of Co-Package 02/10/2025 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 30 Part 2 1 BOTTLE 118 mL Part 3 1 BOTTLE 118 mL Part 4 1 BOTTLE 30 Part 1 of 4 IBUPROFEN
ibuprofen tablet, film coatedProduct Information Item Code (Source) NDC: 73614-201 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IBUPROFEN (UNII: WK2XYI10QM) (IBUPROFEN - UNII:WK2XYI10QM) IBUPROFEN 800 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) STEARIC ACID (UNII: 4ELV7Z65AP) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TALC (UNII: 7SEV7J4R1U) STARCH, CORN (UNII: O8232NY3SJ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white Score no score Shape OVAL (CAPLET) Size 19mm Flavor Imprint Code BI8 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73614-201-03 30 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202413 05/23/2024 Part 2 of 4 CHLORHEXIDINE GLUCONATE ORAL RINSE
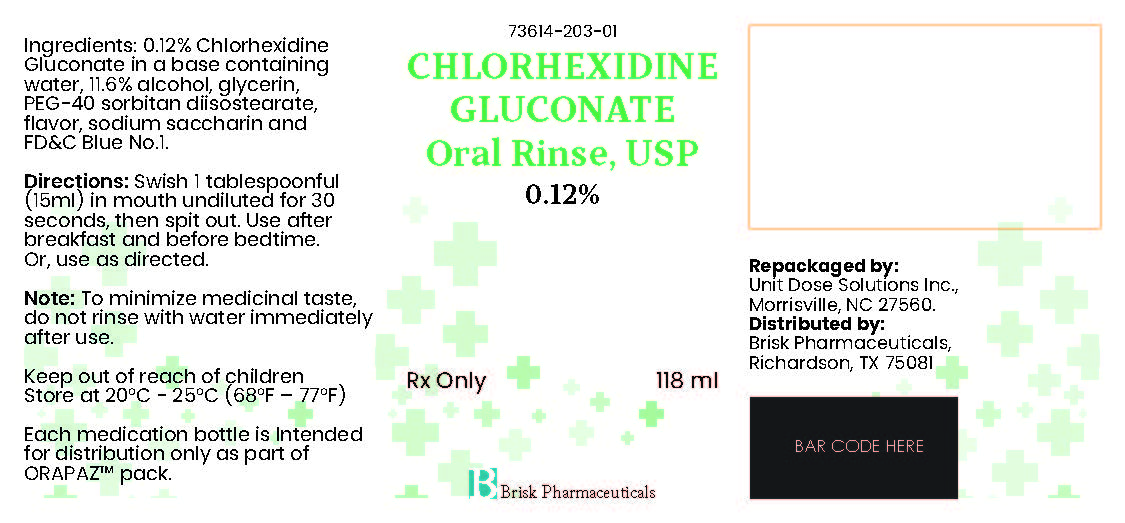
chlorhexidine gluconate oral rinse solutionProduct Information Item Code (Source) NDC: 73614-203 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE GLUCONATE 1.2 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) SACCHARIN SODIUM (UNII: SB8ZUX40TY) PEG-40 SORBITAN DIISOSTEARATE (UNII: JL4CCU7I1G) Product Characteristics Color blue Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73614-203-01 118 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075561 07/27/2023 Part 3 of 4 CHLORHEXIDINE GLUCONATE ORAL RINSE
chlorhexidine gluconate oral rinse solutionProduct Information Item Code (Source) NDC: 73614-203 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE GLUCONATE 1.2 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) SACCHARIN SODIUM (UNII: SB8ZUX40TY) PEG-40 SORBITAN DIISOSTEARATE (UNII: JL4CCU7I1G) Product Characteristics Color blue Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73614-203-01 118 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075561 07/27/2023 Part 4 of 4 CLINDAMYCIN HYDROCHLORIDE
clindamycin hydrochloride capsuleProduct Information Item Code (Source) NDC: 73614-204 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLINDAMYCIN HYDROCHLORIDE (UNII: T20OQ1YN1W) (CLINDAMYCIN - UNII:3U02EL437C) CLINDAMYCIN 150 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, CORN (UNII: O8232NY3SJ) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) MAGNESIUM STEARATE (UNII: 70097M6I30) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) TALC (UNII: 7SEV7J4R1U) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Product Characteristics Color green (LIGHT GREEN) , blue (LIGHT BLUE) Score no score Shape CAPSULE Size 19mm Flavor Imprint Code G;143 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73614-204-03 30 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA216957 03/10/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 02/10/2025 Labeler - Brisk Pharmaceuticals (117250794)
Trademark Results [ClindaCare]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CLINDACARE 76413990 not registered Dead/Abandoned |
Altana, Inc. 2002-05-24 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.