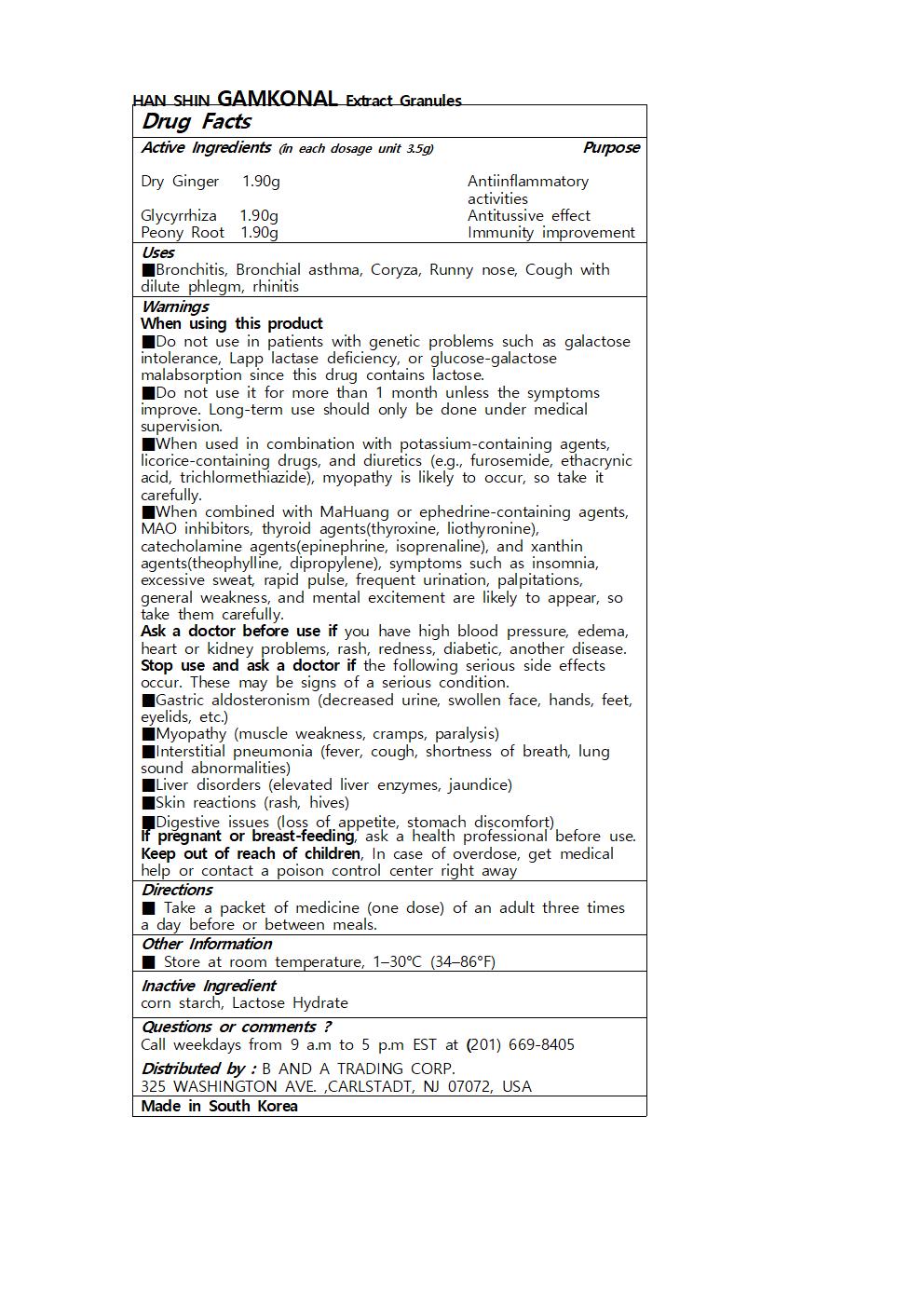
HAN SHIN GAMKONAL EXTRACT GRANULES- dry ginger, glycyrrhiza, peony root granule
HAN SHIN GAMKONAL Extract Granules by
Drug Labeling and Warnings
HAN SHIN GAMKONAL Extract Granules by is a Otc medication manufactured, distributed, or labeled by Lydia Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
WARNINGS
Warnings
When using this product
■Do not use in patients with genetic problems such as galactose intolerance, Lapp lactase deficiency, or glucose-galactose malabsorption since this drug contains lactose.
■Do not use it for more than 1 month unless the symptoms improve. Long-term use should only be done under medical supervision.
■When used in combination with potassium-containing agents, licorice-containing drugs, and diuretics (e.g., furosemide, ethacrynic acid, trichlormethiazide), myopathy is likely to occur, so take it carefully.
■When combined with MaHuang or ephedrine-containing agents, MAO inhibitors, thyroid agents(thyroxine, liothyronine), catecholamine agents(epinephrine, isoprenaline), and xanthin agents(theophylline, dipropylene), symptoms such as insomnia, excessive sweat, rapid pulse, frequent urination, palpitations, general weakness, and mental excitement are likely to appear, so take them carefully.
Ask a doctor before use if you have high blood pressure, edema, heart or kidney problems, rash, redness, diabetic, another disease.
Stop use and ask a doctor if the following serious side effects occur. These may be signs of a serious condition.
■Gastric aldosteronism (decreased urine, swollen face, hands, feet, eyelids, etc.)
■Myopathy (muscle weakness, cramps, paralysis)
■Interstitial pneumonia (fever, cough, shortness of breath, lung sound abnormalities)
■Liver disorders (elevated liver enzymes, jaundice)
■Skin reactions (rash, hives)
■Digestive issues (loss of appetite, stomach discomfort)
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children, In case of overdose, get medical help or contact a poison control center right away
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HAN SHIN GAMKONAL EXTRACT GRANULES
dry ginger, glycyrrhiza, peony root granuleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 72988-0051 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PAEONIA LACTIFLORA ROOT (UNII: 3Z3866YW6P) (PAEONIA LACTIFLORA ROOT - UNII:3Z3866YW6P) PAEONIA LACTIFLORA ROOT 1.9 g in 3.5 g GINGER (UNII: C5529G5JPQ) (GINGER - UNII:C5529G5JPQ) GINGER 1.9 g in 3.5 g LICORICE (UNII: 61ZBX54883) (LICORICE - UNII:61ZBX54883) LICORICE 1.9 g in 3.5 g Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72988-0051-1 120 in 1 PACKAGE 02/01/2025 1 3.5 g in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 02/01/2025 Labeler - Lydia Co., Ltd. (695735569) Registrant - Lydia Co., Ltd. (695735569) Establishment Name Address ID/FEI Business Operations Lydia Co., Ltd. 695735569 manufacture(72988-0051) , label(72988-0051)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.