BARRIER FOR CATS- imidacloprid, moxidectin solution
BARRIER FOR CATS by
Drug Labeling and Warnings
BARRIER FOR CATS by is a Animal medication manufactured, distributed, or labeled by Aurora Pharmaceutical, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
SPL UNCLASSIFIED SECTION
Once-a-month topical solution for cats for the prevention of heartworm disease, kills adult fleas, is indicated for the treatment of flea infestations, as well as the treatment and control of ear mite infestations and intestinal parasite infections in cats and kittens 9 weeks of age and older and that weigh at least 2 lbs.
Once-a-month topical solution for ferrets for the prevention of heartworm disease, kills adult fleas, and is indicated for the treatment of flea infestations. Indicated for ferrets that weigh at least 2 lbs.
CAUTION:
Federal (U.S.A.) Law restricts this drug to use by or on the order of a licensed veterinarian.
-
DESCRIPTION:
Barrier for Cats (10 % imidacloprid + 1 % moxidectin) is a colorless to yellow ready-to-use solution packaged in single-dose applicator tubes for topical treatment of cats. The formulation and dosage schedule are designed to provide a minimum of 4.5 mg/lb (10.0 mg/kg) imidacloprid and 0.45 mg/lb (1.0 mg/kg) moxidectin based on body weight. Imidacloprid is a chloronicotinyl nitroguanidine insecticide. The chemical name of imidacloprid is 1-[(6-Chloro-3-pyridinyl)methyl]-N-nitro-2-imidazolidinimine. Moxidectin is a semisynthetic macrocyclic lactone endectocide derived from the actinomycete Streptomycetes cyaneogriseus noncyanogenus. The chemical name of moxidectin is [6R, 23E, 25S(E)]-5-0-Demethyl-28-deoxy-25-(1,3-dimethyl-1-butenyl)-6,28-epoxy-23-(methoxyimino) milbemycin B.
-
INDICATIONS:
Barrier for Cats is indicated for the prevention of heartworm disease caused by Dirofilaria immitis. Barrier for Cats kills adult fleas (Ctenocephalides felis) and is indicated for the treatment of flea infestations. Barrier for Cats is also indicated for the treatment and control of ear mite (Otodectes cynotis) infestations and the following intestinal parasites:
Intestinal Parasite
Intestinal Stage
Adult
Immature Adult
Fourth
Stage
LarvaeHookworm Species
Ancylostoma tubaeforme
X
X
X
Roundworm Species
Toxocara cati
X
X
-
WARNINGS:
Do not use on sick, debilitated, or underweight cats (See ADVERSE REACTIONS). Do not use on cats less than 9 weeks of age or less than 2 lbs. body weight.
-
HUMAN WARNINGS:
Not for human use. Keep out of the reach of children.
Children should not come in contact with the application site for 30 minutes after application.
Causes eye irritation. Harmful if swallowed. Do not get in eyes or on clothing. Avoid contact with skin. Exposure to the product has been reported to cause headache; dizziness; and redness, burning, tingling, or numbness of the skin.
Wash hands thoroughly with soap and warm water after handling.
If contact with eyes occurs, hold eyelids open and flush with copious amounts of water for 15 minutes. If eye irritation develops or persists, contact a physician. If swallowed, call poison control center or physician immediately for treatment advice. Have person sip a glass of water if able to swallow. Do not induce vomiting unless told to do so by the poison control center or physician. People with known hypersensitivity to benzyl alcohol, imidacloprid or moxidectin should administer the product with caution. In case of allergic reaction, contact a physician. If contact with skin or clothing occurs, take off contaminated clothing. Wash skin immediately with plenty of soap and water. Call a poison control center or physician for treatment advice.
The Safety Data Sheet (SDS) provides additional occupational safety information. For a copy of the Safety Data Sheet (SDS), to report adverse reactions, or consumer questions call Aurora Pharmaceutical at 1-888-215-1256.
-
PRECAUTIONS:
Do not dispense dose applicator tubes without complete safety and administration information. Avoid oral ingestion. Cats may experience hypersalivation, tremors, vomiting and decreased appetite if Barrier for Cats is inadvertently administered orally or through grooming/licking of the application site. The safety of Barrier for Cats has not been established in breeding, pregnant, or lactating cats. The effectiveness of Barrier for Cats against heartworm infections (D. immitis) after bathing has not been evaluated in cats. Use of this product in geriatric patients with subclinical conditions has not been adequately studied. Several otherwise healthy, thin geriatric cats experienced prolonged lethargy and sleepiness after using this drug. (See ADVERSE REACTIONS.)
-
ADVERSE REACTIONS:
Field Study:Following treatment with imidacloprid and moxidectin topical solution or an active control, cat owners reported the following post-treatment reactions:
* These three cats were from the same household and included one 13-yr-old cat in good health, one 15-yr-old FIV positive cat in good health, and one 15-yr-old, underweight cat in fair health. Lethargy was noted for 24 to 36 hrs after the first treatment only; one cat was unsteady at 48 hrs. These cats were not on other medications. OBSERVATION Imidacloprid and Moxidectin Topical Solution n = 113 Active Control
n = 38Lethargy (protracted sleeping, poorly responsive) 3 cats* (2.7 %) None observed Behavioral changes (e.g., agitated, excessive grooming, hiding, pacing, spinning) 9 cats (8.0 %) 1 cat (2.6 %) Discomfort (e.g., scratching, rubbing, head-shaking) 5 cats (4.4 %) None observed Hypersalivation (within 1 hour after treatment) 3 cats (2.7 %) None observed Polydipsia 3 cats (2.7 %) None observed Coughing and gagging 1 cat (0.9 %) None observed During another field study, a 16-year-old cat with renal disease slept in the same place without moving for two days following application. (See PRECAUTIONS.)
Laboratory Effectiveness Studies: Imidacloprid and moxidectin topicl solution was administered at the recommended dose to 215 cats in 20 effectiveness studies. One random-sourced cat exhibited signs consistent with either moxidectin toxicity or viral respiratory disease and died 26 hours after product application; necropsy findings were inconclusive as to the cause of death. A second cat that became ill 3 days after application of imidacloprid and moxidectin topical solution responded to treatment for respiratory infection and completed the study. A third cat became ill on day 3 and died with signs and lesions attributable to panleukopenia on day 7 after moxidectin application.
Post-Approval Experience: The following adverse events are based on post-approval adverse drug experience reporting. Not all adverse reactions are reported to FDA CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using this data. The following adverse events in cats are listed in decreasing order of reporting frequency: hypersalivation, depression/lethargy, application site reactions (alopecia, pruritus, lesions, and erythema), decreased appetite, vomiting, hyperactivity, ataxia, trembling, and behavior disorder (hiding).
In some cases death has been reported.
In humans, ocular and dermal irritation, nausea, numbness or tingling of the mouth and lips, anaphylaxis, pruritus, vomiting, and tongue/taste abnormalities have been reported following exposure to this product.
To report suspected adverse events, for technical assistance or to obtain a copy of the Safety Data Sheet, contact Aurora Pharmaceutical at 1-888-215-1256 or www.aurorapharmaceutical.com.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae.
-
DOSAGE AND ADMINISTRATION:
The recommended minimum dose is 4.5 mg/lb (10.0 mg/kg) imidacloprid and 0.45 mg/lb (1.0 mg/kg) moxidectin, once a month, by topical administration.
Do not apply to irritated skin.
1. Remove one dose applicator tube from the package. As specified in the following table, administer the entire contents of the Barrier for Cats tube that correctly corresponds with the body weight of the cat.
* Cats over 18 lbs. should be treated with the appropriate combination of Barrier for Cats tubes. Cat (lbs.)
Volume (mL)
Imidacloprid (mg)
Moxidectin (mg)
2–5
0.23
23
2.3
5.1–9
0.4
40
4
9.1–18*
0.8
80
8
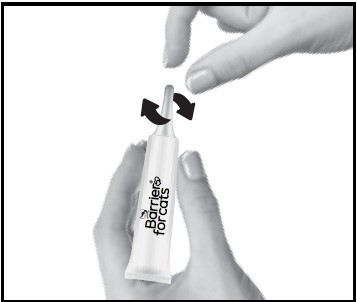
2. While holding the tube in an upright position, twist the cap to break the seal and then apply. The cap will remain on the tube.3.Part the hair on the back of the cat’s neck at the base of the head, until the skin is visible. Place the tip of the tube on the skin and apply the entire contents directly on the exposed skin. Lift tube away from skin before releasing pressure on tube.

3. Part the hair on the back of the cat’s neck at the base of the head, until the skin is visible. Place the tip of the tube on the skin and apply the entire contents directly on the exposed skin. Lift tube away from skin before releasing pressure on tube.
Do not get this product in the cat's mouth or eyes or allow the cat to lick the application site for 30 minutes. Treatment at the base of the head will minimize the opportunity for ingestion by grooming. In households with multiple pets, keep animals separated to prevent licking of the application site.
Stiff, matted hair or a damp, oily appearance of the hair may be observed at the application site on some cats. This is temporary and does not affect the safety and effectiveness of the product.
Heartworm Prevention: For prevention of heartworm disease, Barrier for Cats should be administered at one-month intervals. Barrier for Cats may be administered year-around or at a minimum should start one month before the first expected exposure to mosquitoes and should continue at monthly intervals until one month after the last exposure to mosquitoes. If a dose is missed and a 30-day interval between doses is exceeded, administer Barrier for Cats immediately and resume the monthly dosing schedule. When replacing another heartworm preventative product in a heartworm prevention program, the first treatment with Barrier for Cats should be given within one month of the last dose of the former medication. At the discretion of the veterinarian, cats older than 6 months of age may be tested to determine the presence of existing heartworm infection before treatment with Barrier for Cats (See ADVERSE REACTIONS – Post-Approval Experience).
Flea Treatment: For the treatment of flea infestations, Barrier for Cats should be administered at one-month intervals. If the cat is already infested with fleas when the first dose of Barrier for Cats is administered, adult fleas on the cat will be killed. However, re-infestation from the emergence of pre-existing pupae in the environment may continue to occur for six weeks or longer after treatment is initiated. Cats treated with imidacloprid, including those with pre-existing flea allergy dermatitis have shown clinical improvement as a direct result of elimination of fleas from the cat.
Ear Mite Treatment: For the treatment of ear mites (Otodectes cynotis), Barrier for Cats should be administered once as a single topical dose. Monthly use of Barrier for Cats will control any subsequent ear mite infestations.
Intestinal Nematode Treatment: For the treatment and control of intestinal hookworm infections caused by Ancylostoma tubaeforme (adults, immature adults and fourth stage larvae) and roundworm infections caused by Toxocara cati (adults and fourth stage larvae), Barrier for Cats should be administered once as a single topical dose.
-
ANIMAL SAFETY:
Studies in Kittens: Imidacloprid and moxidectin topical solution was topically applied at 0, 1, 3, and 5X the maximum dose to 48 healthy 9-week-old kittens on days 0, 28, and 56. Lethargy was observed in 1 kitten from the 3X group and 1 from the 5X group on the day after initial treatment; the kitten from the 3X group was also disoriented and ataxic. One kitten from the 3X group had a slow pupillary light response two days after treatment and one had tremors the day after treatment. Hypersalivation was seen in one kitten from the 5X group approximately six hours post-treatment. One kitten from the 3X group was scratching at the treatment site 2 days after treatment. Slight cough was noted in 7 different kittens (2-0X, 2-1X, and 3-5X) during the 13-day period following the first treatment. Histopathology showed granulomatous inflammation at the treatment site in three 1X kittens. Causal relationship to the drug could not be determined. Pulmonary inflammation (1-5X) and lymphoid hyperplasia (2-1X, 4-3X) were seen in treated kittens. In a second study, imidacloprid and moxidectin topical solution was topically applied at 0, 1.7, 5.2 and 8.7X the maximum dose to 48 healthy 9-week-old kittens every two weeks for 6 doses. One kitten in the 8.7X group apparently ingested an unknown amount of the drug and developed the following clinical signs prior to euthanasia: mydriasis, salivation, depression, vomiting, unsteadiness, rapid to slow to difficult breathing, poor pupillary response, generalized tremors, inability to move, and nystagmus. Two kittens in the 5.2X group developed mydriasis, salivation, depression, squinting, and poor appetite. A kitten in the 1.7X group developed mydriasis.
Dose Tolerance Study: Eight healthy juvenile cats were topically dosed with a single application of imidacloprid and moxidectin topical solution at 10 times the recommended dose volume. Mild, transient hypersalivation occurred in two of the cats.
Oral Study in Cats: The oral safety of imidacloprid and moxidectin topical solution was tested in case of accidental oral ingestion. The maximum topical dose was orally administered to twelve healthy 9-week-old kittens. Hypersalivation (8 of 12 kittens) and vomiting (12 of 12 kittens) were observed immediately post-treatment. Tremors developed in one kitten within 1 hour, resolving without treatment within the next hour. All 12 kittens were either anorexic or had decreased appetite for at least 1 day following treatment. In 3 kittens, the anorexia or decreased appetite continued into the second week following treatment. There was a post-treatment loss of body weight in treated kittens compared to control kittens. In a pilot safety study using kittens younger in age and lighter in weight than allowed by product labeling, an 8-week old kitten weighing 0.6 kg orally received 2X of the label topical dose (0.46 mL/kg). Immediately after dosing, it vomited, had labored breathing and slight tremors. Within 4 hours, it was normal, but was found dead on day 6. Necropsy could not determine the cause of death.
Study in Heartworm Positive Cats: Young adult cats were inoculated subcutaneously with third-stage D. immitis larvae. At 243–245 days post-infection, immunoserology and echocardiography were performed to identify cats with adult heartworm burdens similar to naturally-acquired infections. Two groups were treated topically with either imidacloprid and moxidectin topical solution at the label dose or placebo, once every 28 days, for three consecutive treatments. A third group was treated topically, once, with imidacloprid and moxidectin topical solution at 5X the label dose. Sporadic vomiting and labored breathing related to heartworm burden were observed in the treatment and control groups. There was no difference between treatment groups in the numbers of adult D. immitis recovered at study conclusion. No adverse reactions were associated with the topical application of imidacloprid and moxidectin topical solution to experimentally heartworm-infected cats.
- FERRETS
- INDICATIONS:
- WARNINGS:
-
PRECAUTIONS:
Do not dispense dose applicator tubes without complete safety and administration information.
The safety of Barrier for Cats has not been established in breeding, pregnant, and lactating ferrets.
Treatment of ferrets weighing less than 2.0 lbs (0.9 kg) should be based on a risk-benefit assessment.
The effectiveness of Barrier for Cats in ferrets weighing over 4.4 lbs (2.0 kg) has not been established.
-
ADVERSE REACTIONS:
Field Safety Study in Ferrets: Imidacloprid and moxidectin topical solution was topically administered to 131 client-owned ferrets at the recommended dose volume (0.4 mL). The ferrets ranged in age from 3 months to 7 years, and weighed between 0.5 and 1.86 kg (1.1 to 4.1 lbs). The dose of imidacloprid ranged between 21.5 to 80.2 mg/kg in this study. The dose of moxidectin ranged between 2.2 to 8.0 mg/kg in this study. Adverse reactions in ferrets following treatment included: pruritus/scratching, scabbing, redness, wounds and inflammation at the treatment site; lethargy; and chemical odor. These adverse reactions resolved without additional therapy. Owners also reported stiffening of the hair at the treatment site, however, this is expected with application of a topical product and is not considered an adverse reaction. Three human adverse reactions were reported. An owner's finger became red following skin contact with the product. One owner reported a headache caused by the chemical odor of the product. One owner reported a tingling sensation of the lips after kissing the treatment site.
Foreign Market Experience: Because the following events were reported voluntarily during post-approval use of the product in foreign markets, it is not always possible to reliably establish a causal relationship to drug exposure. Adverse events reported in ferrets topically treated with 0.4 mL imidacloprid + moxidectin for cats included: malaise, vomiting, diarrhea, shaking, mydriasis, hypersalivation with abnormal neurologic signs, seizures, death, generalized hematoma of the body, and alopecia at the treatment site. Adverse reactions in humans included: burning, tingling, numbness, bad taste in the mouth, dizziness, and headache.
-
ANIMAL SAFETY:
Ferrets: Imidacloprid and moxidectin topical solution was topically applied at 5X the recommended dose volume to six healthy 9-month-old ferrets on Study Days 0, 14, 28, and 42. Because the weights of the ferrets in this study ranged from 2.0 to 4.0 lb (0.9 kg to 1.8 kg), ferrets received a range of dosages from 51.0 to 106.9 mg/lb (112 to 235 mg/kg) of imidacloprid and 5 to 10.5 mg/lb (11 to 23 mg/kg) of moxidectin. The following abnormal clinical signs were reported during the study: wet, matted, and/or greasy appearance to the hair, shaking of the head and/or body, rubbing of dose site on cage, and shedding. Slight increases in phosphorous, potassium, aspartate aminotransferase (AST), and glucose were seen during the study, however, no clinical signs related to these bloodwork changes were reported.
Oral Safety Study: Imidacloprid and moxidectin topical solution was orally administered at the recommended dose volume (0.4 mL) to eight healthy ferrets on Study Day 0. Ferrets were 78 to 101 days old (11.1 to 14.4 weeks) and weighed between 1.1 to 1.8 lb (0.5 to 0.8 kg) body weight on the day of dosing, resulting in doses ranges of 22.0–36.8 mg/lb (48.3–81.0 mg/kg) imidacloprid and 2.2–3.7 mg/lb (4.8–8.0 mg/kg) moxidectin. The following abnormal clinical signs were reported immediately following oral administration of imidacloprid and moxidectin topical solution: vomiting (one ferret) and ataxia (two ferrets). All abnormalities resolved without treatment or supportive care.
-
DOSAGE AND ADMINISTRATION:
For ferrets:
The recommended minimum dose for a ferret is 9 mg/lb (20 mg/kg) imidacloprid and 0.9 mg/lb (2 mg/kg) moxidectin, once a month, by topical administration.
Ferret (lbs.)
Barrier for Cats
Volume (mL)
Imidacloprid (mg)
Moxidectin (mg)
2.0–4.4
Barrier for Cats 9
0.4
40
4
Only the 0.4 mL applicator tube volume (Barrier for Cats 9) should be used on ferrets.
Do not apply to irritated skin.
1. Remove one dose applicator tube from the package. Administer the entire contents of the Barrier for Cats tube (0.4 mL).
2. While holding the tube in an upright position, twist the cap to break the seal and then apply. The cap will remain on the tube.

3. Part the hair on the back of the ferret's neck at the base of the head, until the skin is visible. Place the tip of the tube on the skin and apply the entire contents directly on the exposed skin. Lift tube away from skin before releasing pressure on tube.
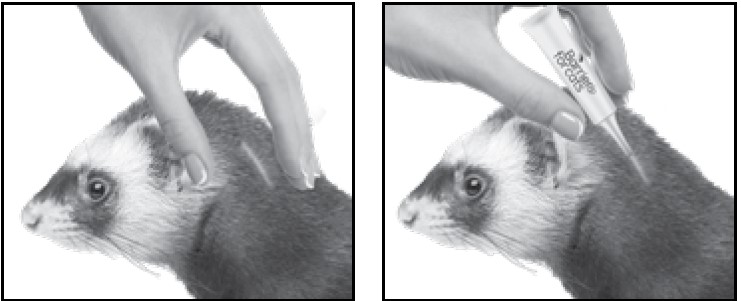
Do not get this product in the ferret's mouth or eyes or allow the ferret to lick the application site for 30 minutes. Treatment at the base of the head will minimize the opportunity for ingestion by grooming. In households with multiple pets, keep animals separated to prevent licking of the application site.
Stiff, matted hair or a damp, oily appearance of the hair may be observed at the application site on some ferrets. This is temporary and does not affect the safety or effectiveness of the product.
Heartworm Prevention: For prevention of heartworm disease, Barrier for Cats should be administered at one-month intervals. Barrier for Cats may be administered year-round or at a minimum should start one month before the first expected exposure to mosquitoes and should continue at monthly intervals until one month after the last exposure to mosquitoes. If a dose is missed and a 30-day interval between doses is exceeded, administer Barrier for Cats immediately and resume the monthly dosing schedule.
Flea Treatment: For the treatment of flea infestations on ferrets, Barrier for Cats should be administered at one-month intervals. If the ferret is already infested with fleas when the first dose of Barrier for Cats is administered, adult fleas on the ferret will be killed. However, re-infestation from the emergence of pre-existing pupae in the environment may continue to occur for six weeks or longer after treatment is initiated.
- STORAGE INFORMATION:
- HOW SUPPLIED:
-
Principal Display Panel - 0.23 mL Carton Label
2 – 5 lbs
Heartworm
Fleas
Hookworm
Roundworm
Ear Mites
3 Pack
Barrier®for cats
(imidacloprid+moxidectin)
Topical Solution
CAUTION: Federal (U.S.A.) Law restricts this drug to use by or on the order of a licensed veterinarian
Once-A-Month Topical Solution
- Prevention of heartworm disease
- Kills adult fleas and is indicated for the treatment of flea infestations
- Treatment and control of ear mite infestations
- Treatment and control of hookworms and roundworms
- For cats and kittens 9 weeks of age and older and 2 –5 lbs.
Children should not come in contact with the application site for 30 minutes after application.
Do not use the 0.23 mL size tube in ferrets as it does not provide an effective dose.
Each tube contains 23 mg of imidacloprid and 2.3 mg of moxidectin.
Keep this and all drugs out of reach of children.
Approved by FDA under ANADA # 200-714
THREE 0.23 mL Tubes
NDC: 51072-101-01
IN 40-1551 06/2025

-
Principal Display Panel - 0.4 mL Carton Label
5.1 – 9 lbs
for
cats:Heartworm
Fleas
Hookworm
Roundworm
Ear Mites
for
ferrets:Heartworm
Fleas
6 Pack
Barrier®for cats
(imidacloprid+moxidectin)
Topical Solution for Cats and Ferrets
CAUTION: Federal (U.S.A.) Law restricts this drug to use by or on the order of a licensed veterinarian
Once-A-Month Topical Solution
For Cats:
- Prevention of heartworm disease
- Kills adult fleas and is indicated for the treatment of flea infestations
- Treatment and control of ear mite infestations
- Treatment and control of hookworms and roundworms
- For cats and kittens 9 weeks of age and olderand 5.1 – 9 lbs.
For Ferrets:
- Prevention of heartworm disease
- Kills adult fleas and treats flea infestations
- For ferrets that weigh at least 2 lbs.
Children should not come in contact with the application site for 30 minutes after application.
Each tube contains 40 mg of imidacloprid and 4 mg of moxidectin.
Keep this and all drugs out of reach of children.
Approved by FDA under ANADA # 200-714
SIX 0.4 mL Tube
NDC: 51072-101-02
IN 40-1552 06/2025

-
Principal Display Panel - 0.8 mL Carton Label
9.1 - 18 lbs
Heartworm
Fleas
Hookworm
Roundworm
Ear Mites
6 Pack
Barrier®for cats
(imidacloprid+moxidectin)
Topical Solution
CAUTION: Federal (U.S.A.) Law restricts this drug to use by or on the order of a licensed veterinarian
Once-A-Month Topical Solution
- Prevention of heartworm disease
- Kills adult fleas and is indicated for the treatment of flea infestations
- Treatment and control of ear mite infestations
- Treatment and control of hookworms and roundworms
- For cats and kittens 9 weeks of age and older and 9.1 –18 lbs.
Children should not come in contact with the application site for 30 minutes after application.
Do not use the 0.8 mL size tube in ferrets as it could result in an overdose.
Each tube contains 80 mg of imidacloprid and 8 mg of moxidectin.
Keep this and all drugs out of reach of children.
Approved by FDA under ANADA # 200-714
SIX 0.8 mL Tubes
NDC: 51072-101-03
IN 40-1553 06/2025

-
INGREDIENTS AND APPEARANCE
BARRIER FOR CATS
imidacloprid, moxidectin solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 51072-101 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IMIDACLOPRID (UNII: 3BN7M937V8) (IMIDACLOPRID - UNII:3BN7M937V8) IMIDACLOPRID 100 mg in 1 mL MOXIDECTIN (UNII: NGU5H31YO9) (MOXIDECTIN - UNII:NGU5H31YO9) MOXIDECTIN 10 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51072-101-01 3 in 1 CARTON 1 0.23 mL in 1 TUBE 2 NDC: 51072-101-02 6 in 1 CARTON 2 0.4 mL in 1 TUBE 3 NDC: 51072-101-03 6 in 1 CARTON 3 0.8 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200714 10/26/2022 Labeler - Aurora Pharmaceutical, Inc (832848639) Establishment Name Address ID/FEI Business Operations Aurora Pharmaceutical, Inc 832848639 manufacture
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.