Major DOK (Docusate Sodium) 100 mg
DOK by
Drug Labeling and Warnings
DOK by is a Otc medication manufactured, distributed, or labeled by Major Pharmaceuticals. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
DOK- docusate sodium tablet, film coated
Major Pharmaceuticals
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
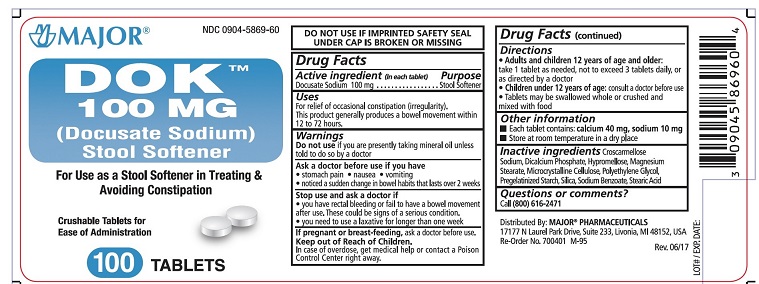
Major DOK (Docusate Sodium) 100 mg
Uses
For relief of occasional constipation (irregularity).
This product generally produces a bowel movement within 12 to 72 hours.
Warnings
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that last over 2 weeks
Directions
- Adults and children 12 years of age and older: take 1 tablet as needed, not to exceed 3 tablets daily, or as directed by a doctor
- Children under 12 years of age: consult a doctoer before use
- Tablets may be swallowed whole or crushed and mixed with food
Other information
- Each tablet contains calcium 40 mg, s odium 10 mg
- Store at room temperature in a dry place
Inactive ingredients Croscarmellose Sodium, Dicalcium Phosphate, Hypromellose, Magnesium Stearate, Microcrystalline Cellulose, Polyethylene Glycol, Pregelatinized Starch, Silica, Sodium Benzoate, Stearic acid
Distributed by: MAJOR® PHARMACEUTICALS
17177 N. Laurel Park Drive, Suite 233, Livonia, MI 48152, USA
MAJOR
®
NDC: 0904-5869-60
DOK
TM
100 MG
(Docusate Sodium)
Stool Softener
For Use as a Stool Softener in Treating and Avoiding Constipation
Crushable Tablets for Ease of Administration
100 TABLETS
| DOK
docusate sodium tablet, film coated |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Major Pharmaceuticals (191427277) |