Live Betr LLC Pain Relief Drug Facts
betr pain relief by
Drug Labeling and Warnings
betr pain relief by is a Otc medication manufactured, distributed, or labeled by Praxis, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
BETR PAIN RELIEF- acetaminophen tablet
Sixarp, LLC
----------
Live Betr LLC Pain Relief Drug Facts
Uses
- temporarily relieves minor aches and pains due to:
- the common cold
- headache
- minor pain of arthritis
- backache
- muscular aches
- toothache
- premenstrual and menstrual cramps
- temporarily reduces fever
Warnings
Liver warning:This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert:Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you have ever had an allergic reaction to this product or any of its
Directions
- do not take more than directed (see overdose warning)
|
adults and children 12 years and over |
|
|
children under 12 years |
ask a doctor |
Inactive ingredients
carnauba wax, corn starch*, croscarmellose sodium*, hypromellose, polyethylene glycol, povidone, pregelatinized starch, sodium starch glycolate*, stearic acid
*may contain one or more of these ingredients
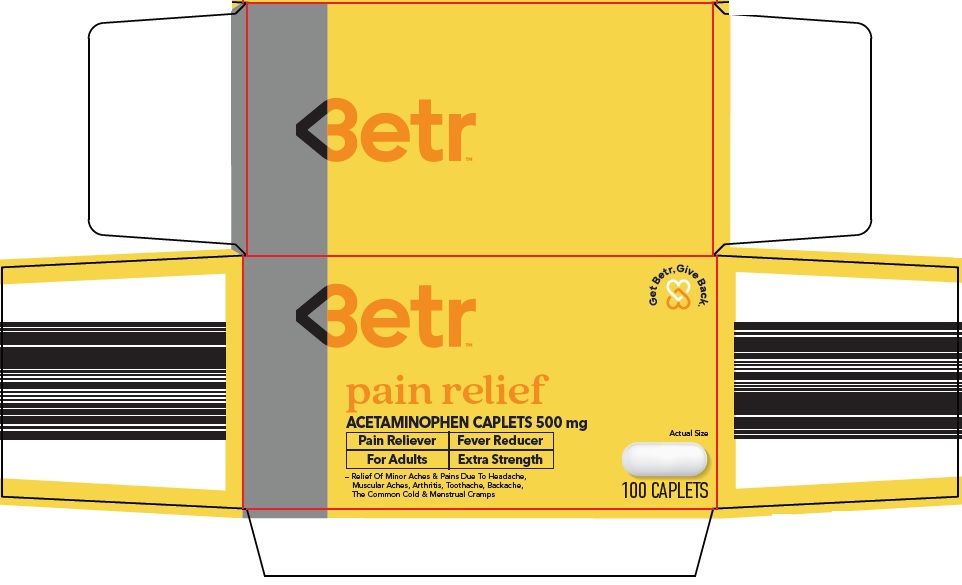
Principal Display Panel
Get Betr, Give Back.
Betr ™
pain relief
ACETAMINOPHEN CAPLETS 500 mg
Actual Size
Pain Reliever | Fever Reducer
For Adults | Extra Strength
- Relief Of Minor Aches & Pains Due To Headache, Muscular Aches, Arthritis, Toothache, Backache, The Common Cold & Menstrual Cramps
100 CAPLETS
| BETR PAIN RELIEF
acetaminophen tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Sixarp, LLC (016329513) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sixarp, LLC | 016329513 | manufacture(59368-217) , pack(59368-217) , label(59368-217) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.