MESNEX- mesna injection, solution
MESNEX by
Drug Labeling and Warnings
MESNEX by is a Prescription medication manufactured, distributed, or labeled by Baxter Healthcare Corporation, Baxter Oncology GmbH. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use MESNEX safely and effectively. See full prescribing information for MESNEX.
MESNEX (mesna) injection, for intravenous use
MESNEX (mesna) tablets, for oral use
Initial U.S. Approval: 1988INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
MESNEX may be given on a fractionated dosing schedule of three bolus intravenous injections or a single bolus injection followed by two oral administrations of MESNEX Tablets as outlined below. The dosing schedule should be repeated on each day that ifosfamide is administered. When the dosage of ifosfamide is adjusted, the ratio of MESNEX to ifosfamide should be maintained. (2)
Intravenous Dosing Schedule:
0 Hours
4 Hours
8 Hours
Ifosfamide
1.2 g/m2
--
--
MESNEX Injection
240 mg/m2
240 mg/m2
240 mg/m2
Intravenous and Oral Dosing Schedule:
0 Hours
2 Hours
6 Hours
Ifosfamide
1.2 g/m2
--
--
MESNEX Injection
240 mg/m2
--
--
MESNEX Tablets
--
480 mg/m2
480 mg/m2
Maintain sufficient urinary output, as required for ifosfamide treatment, and monitor urine for the presence of hematuria. (2.3)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
Known hypersensitivity to MESNEX or to any of the excipients, including benzyl alcohol. (4)
WARNINGS AND PRECAUTIONS
- Hypersensitivity reactions: Anaphylactic reactions have been reported. Less severe hypersensitivity reactions may also occur. Monitor patients. If a reaction occurs, discontinue MESNEX and provide supportive care. (5.1)
- Dermatologic toxicity: Skin rash with eosinophilia and systemic symptoms, Stevens-Johnson syndrome, and toxic epidermal necrolysis have occurred. Skin rash, urticaria, and angioedema have also been seen. Monitor patients. If a reaction occurs, discontinue MESNEX and provide supportive care. (5.2)
- Benzyl alcohol toxicity: The preservative benzyl alcohol has been associated with serious adverse reactions and death in neonates and premature infants. Avoid use in neonates, premature, and low-birth weight infants. (5.3)
- Laboratory test alterations: False positive tests for urinary ketones and interference with enzymatic CPK activity tests have been seen. (5.4)
ADVERSE REACTIONS
The most common adverse reactions (> 10%) when MESNEX is given with ifosfamide are nausea, vomiting, constipation, leukopenia, fatigue, fever, anorexia, thrombocytopenia, anemia, granulocytopenia, diarrhea, asthenia, abdominal pain, headache, alopecia, and somnolence. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Baxter Healthcare at 1-866-888-2472, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Intravenous Dosing
2.2 Intravenous and Oral Dosing
2.3 Monitoring for Hematuria
2.4 Preparation for Intravenous Administration and Stability
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Dermatologic Toxicity
5.3 Benzyl Alcohol Toxicity
5.4 Laboratory Test Interferences
5.5 Use in Patients with a History of Adverse Reactions to Thiol Compounds
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Use in Patients with Renal Impairment
8.7 Use in Patients with Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Intravenous MESNEX
14.2 Oral MESNEX
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Intravenous Dosing
MESNEX may be given on a fractionated dosing schedule of three bolus intravenous injections as outlined below.
MESNEX injection is given as intravenous bolus injections in a dosage equal to 20% of the ifosfamide dosage weight by weight (w/w) at the time of ifosfamide administration and 4 and 8 hours after each dose of ifosfamide. The total daily dose of MESNEX is 60% of the ifosfamide dose. The recommended dosing schedule is outlined below in .
- * The dosing schedule should be repeated on each day that ifosfamide is administered. When the dosage of ifosfamide is increased or decreased, the ratio of MESNEX to ifosfamide should be maintained.
Table 1. Recommended Intravenous Dosing Schedule
0 Hours
4 Hours
8 Hours
Ifosfamide
1.2 g/m2
-
-
MESNEX Injection*
240 mg/m2
240 mg/m2
240 mg/m2
2.2 Intravenous and Oral Dosing
MESNEX may be given on a fractionated dosing schedule of a single bolus injection followed by two oral administrations of MESNEX tablets as outlined below.
MESNEX injection is given as intravenous bolus injections in a dosage equal to 20% of the ifosfamide dosage (w/w) at the time of ifosfamide administration. MESNEX tablets are given orally in a dosage equal to 40% of the ifosfamide dose 2 and 6 hours after each dose of ifosfamide. The total daily dose of MESNEX is 100% of the ifosfamide dose. The recommended dosing schedule is outlined in .
- * The dosing schedule should be repeated on each day that ifosfamide is administered. When the dosage of ifosfamide is increased or decreased, the ratio of MESNEX to ifosfamide should be maintained.
Table 2. Recommended Intravenous and Oral Dosing Schedule
0 Hours
2 Hours
6 Hours
Ifosfamide
1.2 g/m2
-
-
MESNEX injection*
240 mg/m2
-
-
MESNEX tablets
-
480 mg/m2
480 mg/m2
The efficacy and safety of this ratio of intravenous and oral MESNEX has not been established as being effective for daily doses of ifosfamide higher than 2 g/m2.
Patients who vomit within two hours of taking oral MESNEX should repeat the dose or receive intravenous MESNEX.
2.3 Monitoring for Hematuria
Maintain adequate hydration and sufficient urinary output, as required for ifosfamide treatment, and monitor urine for the presence of hematuria. If severe hematuria develops when MESNEX is given according to the recommended dosage schedule, dosage reductions or discontinuation of ifosfamide therapy may be required.
2.4 Preparation for Intravenous Administration and Stability
Preparation
Determine the volume of MESNEX injection for the intended dose.
Dilute the volume of MESNEX injection for the dose in any of the following fluids to obtain a final concentration of 20 mg/mL:
- 5% Dextrose Injection, USP
- 5% Dextrose and 0.2% Sodium Chloride Injection, USP
- 5% Dextrose and 0.33% Sodium Chloride Injection, USP
- 5% Dextrose and 0.45% Sodium Chloride Injection, USP
- 0.9% Sodium Chloride Injection, USP
- Lactated Ringer’s Injection, USP
Stability
The MESNEX injection multidose vials may be stored and used for up to 8 days after initial puncture.
Store diluted solutions at 25°C (77°F). Use diluted solutions within 24 hours.
Do not mix MESNEX injection with epirubicin, cyclophosphamide, cisplatin, carboplatin, and nitrogen mustard.
The benzyl alcohol contained in MESNEX injection vials can reduce the stability of ifosfamide. Ifosfamide and MESNEX may be mixed in the same bag provided the final concentration of ifosfamide does not exceed 50 mg/mL. Higher concentrations of ifosfamide may not be compatible with MESNEX and may reduce the stability of ifosfamide.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. Any solutions which are discolored, hazy, or contain visible particulate matter should not be used.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
MESNEX is contraindicated in patients known to be hypersensitive to MESNEX or to any of the excipients [see Warnings and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
MESNEX may cause systemic hypersensitivity reactions, including anaphylaxis. These reactions may include fever, cardiovascular symptoms (hypotension, tachycardia), acute renal impairment, hypoxia, respiratory distress, urticaria, angioedema, laboratory signs of disseminated intravascular coagulation, hematological abnormalities, increased liver enzymes, nausea, vomiting, arthralgia, and myalgia. These reactions may occur with the first exposure or after several months of exposure. Monitor for signs or symptoms. Discontinue MESNEX and provide supportive care.
5.2 Dermatologic Toxicity
Drug rash with eosinophilia and systemic symptoms and bullous and ulcerative skin and mucosal reactions, consistent with Stevens-Johnson syndrome or toxic epidermal necrolysis have occurred. MESNEX may cause skin and mucosal reactions characterized by urticaria, rash, erythema, pruritus, burning sensation, angioedema, periorbital edema, flushing and stomatitis. These reactions may occur with the first exposure or after several months of exposure. Discontinue MESNEX and provide supportive care.
5.3 Benzyl Alcohol Toxicity
Benzyl alcohol, a preservative in MESNEX, has been associated with serious adverse reactions and death (including gasping syndrome) in neonates, premature, and low-birth weight infants. The minimum amount of benzyl alcohol at which toxicity may occur is not known. Consider the combined daily metabolic load of benzyl alcohol from all sources when prescribing MESNEX (10.4 mg benzyl alcohol per mL). Neonates, premature, and low-birth weight infants, as well as patients receiving high dosages, may be more likely to develop toxicity. Monitor patients for signs or symptoms of toxicity. Avoid use in neonates, premature, and low-birth weight infants [See Use in Specific Populations (8.4)].
5.4 Laboratory Test Interferences
False-Positive Urine Tests for Ketone Bodies
A false positive test for urinary ketones may arise in patients treated with MESNEX when using nitroprusside sodium-based urine tests (including dipstick tests). The addition of glacial acetic acid can be used to differentiate between a false positive result (cherry-red color that fades) and a true positive result (red-violet color that intensifies).
False-Negative Tests for Enzymatic CPK Activity
MESNEX may interfere with enzymatic creatinine phosphokinase (CPK) activity tests that use a thiol compound (e.g., N-acetylcysteine) for CPK reactiviation. This may result in a falsely low CPK level.
False-Positive Tests for Ascorbic Acid
MESNEX may cause false-positive reactions in Tillman’s reagent-based urine screening tests for ascorbic acid.
5.5 Use in Patients with a History of Adverse Reactions to Thiol Compounds
MESNEX is a thiol compound, i.e., a sulfhydryl (SH) group-containing organic compound. Hypersensitivity reactions to MESNEX and to amifostine, another thiol compound, have been reported. It is not clear whether patients who experienced an adverse reaction to a thiol compound are at increased risk for a hypersensitivity reaction to MESNEX.
-
6 ADVERSE REACTIONS
The following are discussed in more detail in other sections of the labeling.
- Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
- Dermatological Toxicity [see Warnings and Precautions (5.2)]
- Benzyl AlcoholToxicity [see Warnings and Precautions (5.3)]
- Laboratory Test Interferences [see Warnings and Precautions (5.4)]
- Use in Patients with a History of Adverse Reactions to Thiol Compounds [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
MESNEX adverse reaction data are available from four Phase 1 studies in which single intravenous doses of 600-1200 mg MESNEX Injection without concurrent chemotherapy were administered to a total of 53 healthy volunteers and single oral doses of 600-2400 mg of MESNEX Tablets were administered to a total of 82 healthy volunteers. The most frequently reported side effects (observed in two or more healthy volunteers) for healthy volunteers receiving single doses of MESNEX Injection alone were headache, injection site reactions, flushing, dizziness, nausea, vomiting, somnolence, diarrhea, anorexia, fever, pharyngitis, hyperesthesia, influenza-like symptoms, and coughing. In two Phase 1 multiple-dose studies where healthy volunteers received MESNEX Tablets alone or intravenous MESNEX followed by repeated doses of MESNEX Tablets, flatulence and rhinitis were reported. In addition, constipation was reported by healthy volunteers who had received repeated doses of intravenous MESNEX.
Additional adverse reactions in healthy volunteers receiving MESNEX alone included injection site reactions, abdominal pain/colic, epigastric pain/burning, mucosal irritation, lightheadedness, back pain, arthralgia, myalgia, conjunctivitis, nasal congestion, rigors, paresthesia, photophobia, fatigue, lymphadenopathy, extremity pain, malaise, chest pain, dysuria, pleuritic pain, dry mouth, dyspnea, and hyperhidrosis. In healthy volunteers, MESNEX was commonly associated with a rapid (within 24 hours) decrease in lymphocyte count, which was generally reversible within one week of administration.
Because MESNEX is used in combination with ifosfamide or ifosfamide-containing chemotherapy regimens, it is difficult to distinguish the adverse reactions which may be due to MESNEX from those caused by the concomitantly administered cytotoxic agents.
Adverse reactions reasonably associated with MESNEX administered intravenously and orally in four controlled studies in which patients received ifosfamide or ifosfamide-containing regimens are presented in .
- * Intravenous dosing of ifosfamide and MESNEX followed by either intravenous or oral doses of MESNEX according to the applicable dosage schedule. [see Dosage and Administration (2)].
Table 3: Adverse Reactions in ≥ 5% of Patients Receiving MESNEX in combination
with Ifosfamide-containing RegimensMESNEX Regimen
Intravenous-Intravenous-Intravenous*
Intravenous-Oral-Oral*
N exposed
119 (100.0%)
119 (100%)
Incidence of AEs
101 (84.9%)
106 (89.1%)
Nausea
65 (54.6)
64 (53.8)
Vomiting
35 (29.4)
45 (37.8)
Constipation
28 (23.5)
21 (17.6)
Leukopenia
25 (21.0)
21 (17.6)
Fatigue
24 (20.2)
24 (20.2)
Fever
24 (20.2)
18 (15.1)
Anorexia
21 (17.6)
19 (16.0)
Thrombocytopenia
21 (17.6)
16 (13.4)
Anemia
20 (16.8)
21 (17.6)
Granulocytopenia
16 (13.4)
15 (12.6)
Asthenia
15 (12.6)
21 (17.6)
Abdominal Pain
14 (11.8)
18 (15.1)
Alopecia
12 (10.1)
13 (10.9)
Dyspnea
11 (9.2)
11 (9.2)
Chest Pain
10 (8.4)
11 (9.2)
Hypokalemia
10 (8.4)
11 (9.2)
Diarrhea
9 (7.6)
17 (14.3)
Dizziness
9 (7.6)
5 (4.2)
Headache
9 (7.6)
13 (10.9)
Pain
9 (7.6)
10 (8.4)
Sweating Increased
9 (7.6)
2 (1.7)
Back Pain
8 (6.7)
6 (5.0)
Hematuria
8 (6.7)
7 (5.9)
Injection Site Reaction
8 (6.7)
10 (8.4)
Edema
8 (6.7)
9 (7.6)
Edema Peripheral
8 (6.7)
8 (6.7)
Somnolence
8 (6.7)
12 (10.1)
Anxiety
7 (5.9)
4 (3.4)
Confusion
7 (5.9)
6 (5.0)
Face Edema
6 (5.0)
5 (4.2)
Insomnia
6 (5.0)
11 (9.2)
Coughing
5 (4.2)
10 (8.4)
Dyspepsia
4 (3.4)
6 (5.0)
Hypotension
4 (3.4)
6 (5.0)
Pallor
4 (3.4)
6 (5.0)
Dehydration
3 (2.5)
7 (5.9)
Pneumonia
2 (1.7)
8 (6.7)
Tachycardia
1 (0.8)
7 (5.9)
Flushing
1 (0.8)
6 (5.0)
6.2 Postmarketing Experience
The following adverse reactions have been reported in the postmarketing experience of patients receiving MESNEX in combination with ifosfamide or similar drugs, making it difficult to distinguish the adverse reactions which may be due to MESNEX from those caused by the concomitantly administered cytotoxic agents. Because these reactions are reported from a population of unknown size, precise estimates of frequency cannot be made.
Cardiovascular: Hypertension
Gastrointestinal: Dysgeusia
Hepatobiliary: Hepatitis
Nervous System: Convulsion
Respiratory: Hemoptysis
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B
Risk Summary
There are no studies of MESNEX in pregnant women. Reproduction studies performed in rats and rabbits at oral doses approximately 10 times the maximum recommended total daily intravenous-oral-oral human dose on a body surface area basis (1000 mg/kg in rabbits and 2000 mg/kg in rats) revealed no evidence of harm to the fetus due to mesna. The incidence of malformations in human pregnancies has not been established for MESNEX. All pregnancies, regardless of drug exposure, have a background rate of 2 to 4% for major malformations and 15 to 20% for pregnancy loss. Because animal reproductive studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
8.3 Nursing Mothers
It is not known whether mesna or dimesna is excreted in human milk. Benzyl alcohol present in maternal serum is likely to cross into human milk and may be orally absorbed by a nursing infant. Because many drugs are excreted in human milk and because of the potential for adverse reactions in nursing infants from MESNEX, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
Safety and effectiveness of MESNEX in pediatric patients have not been established. MESNEX contains benzyl alcohol (10.4 mg benzyl alcohol per mL) which has been associated with serious adverse reactions and death in pediatric patients. The "gasping syndrome," (characterized by central nervous system depression, metabolic acidosis and gasping respirations) has been associated with benzyl alcohol dosages >99 mg/kg/day in neonates, premature, and low-birth weight infants. Additional symptoms may include gradual neurological deterioration, seizures, intracranial hemorrhage, hematologic abnormalities, skin breakdown, hepatic and renal failure, hypotension, bradycardia, and cardiovascular collapse. The minimum amount of benzyl alcohol at which toxicity may occur is not known. Neonates, premature, and low-birth weight infants, as well as patients receiving high dosages, may be more likely to develop toxicity. Practitioners administering this and other medications containing benzyl alcohol should consider the combined daily metabolic load of benzyl alcohol from all sources [see Warnings and Precautions (5.3)].
8.5 Geriatric Use
Clinical studies of MESNEX did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. The ratio of ifosfamide to MESNEX should remain unchanged.
-
10 OVERDOSAGE
There is no known antidote for MESNEX.
In a clinical trial, 11 patients received intravenous MESNEX 10 mg/kg to 66 mg/kg per day for 3 to 5 days. Patients also received ifosfamide or cyclophosphamide. Adverse reactions included nausea, vomiting, diarrhea and fever. An increased rate of these adverse reactions has also been found in oxazaphosphorine-treated patients receiving ≥80 mg MESNEX per kg per day intravenously compared with patients receiving lower doses or hydration treatment only.
Postmarketing, administration of 4.5 g to 6.9 g of MESNEX resulted in hypersensitivity reactions including mild hypotension, shortness of breath, asthma exacerbation, rash, and flushing.
-
11 DESCRIPTION
MESNEX is a detoxifying agent to inhibit the hemorrhagic cystitis induced by ifosfamide. The active ingredient, mesna, is a synthetic sulfhydryl compound designated as sodium-2-mercaptoethane sulfonate with a molecular formula of C2H5NaO3S2 and a molecular weight of 164.18. Its structural formula is as follows:
HS–CH2–CH2SO3–Na+
MESNEX (mesna) injection is a sterile, nonpyrogenic, aqueous solution of clear and colorless appearance in clear glass multidose vials for intravenous administration. MESNEX injection contains 100 mg/mL mesna, 0.25 mg/mL edetate disodium and sodium hydroxide for pH adjustment. MESNEX Injection multidose vials also contain 10.4 mg/mL of benzyl alcohol as a preservative. The solution has a pH range of 7.5-8.5.
MESNEX (mesna) tablets are white, oblong, scored biconvex film-coated tablets with the imprint M4. They contain 400 mg mesna. The excipients are calcium phosphate, cornstarch, hydroxypropylmethylcellulose, lactose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone, simethicone, and titanium dioxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Mesna reacts chemically with the urotoxic ifosfamide metabolites, acrolein and 4-hydroxy-ifosfamide, resulting in their detoxification. The first step in the detoxification process is the binding of mesna to 4‑hydroxy-ifosfamide forming a non-urotoxic 4-sulfoethylthioifosfamide. Mesna also binds to the double bonds of acrolein and to other urotoxic metabolites and inhibits their effects on the bladder.
12.3 Pharmacokinetics
Absorption
Following oral administration, peak plasma concentrations were reached within 1.5 to 4 hours and 3 to 7 hours for free mesna and total mesna (mesna plus dimesna and mixed disulfides), respectively. Oral bioavailability averaged 58% (range 45 to 71%) for free mesna and 89% (range 74 to 104%) for total mesna based on plasma AUC data from 8 healthy volunteers who received 1200 mg oral or intravenous doses.
Food does not affect the urinary availability of orally administered MESNEX.
Distribution
Mean apparent volume of distribution (Vd) for mesna is 0.652 ± 0.242 L/kg after intravenous administration which suggests distribution to total body water (plasma, extracellular fluid, and intracellular water).
Metabolism
Analogous to the physiological cysteine-cystine system, mesna is rapidly oxidized to its major metabolite, mesna disulfide (dimesna). Plasma concentrations of mesna exceed those of dimesna after oral or intravenous administration.
Excretion
Following intravenous administration of a single 800 mg dose, approximately 32% and 33% of the administered dose was eliminated in the urine in 24 hours as mesna and dimesna, respectively. Mean plasma elimination half-lives of mesna and dimesna are 0.36 hours and 1.17 hours, respectively. Mesna has a plasma clearance of 1.23 L/h/kg.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term studies in animals have been performed to evaluate the carcinogenic potential of mesna.
Mesna was not genotoxic in the in vitro Ames bacterial mutagenicity assay, the in vitro mammalian lymphocyte chromosomal aberration assay or the in vivo mouse micronucleus assay.
No studies on male or female fertility were conducted. No signs of male or female reproductive organ toxicity were seen in 6-month oral rat studies (≤ 2000 mg/kg/day) or 29-week oral dog studies (520 mg/kg/day) at doses approximately 10-fold higher than the maximum recommended human dose on a body surface area basis.
-
14 CLINICAL STUDIES
14.1 Intravenous MESNEX
Hemorrhagic cystitis produced by ifosfamide is dose dependent (Table 4). At a dose of 1.2 g/m2 ifosfamide administered daily for 5 days, 16 to 26% of the patients who received conventional uroprophylaxis (high fluid intake, alkalinization of the urine, and the administration of diuretics) developed hematuria (>50 RBC per hpf or macrohematuria) (Studies 1, 2, and 3). In contrast, none of the patients who received mesna injection together with this dose of ifosfamide developed hematuria (Studies 3 and 4). In two randomized studies, (Studies 5 and 6), higher doses of ifosfamide, from 2 g/m2 to 4 g/m2 administered for 3 to 5 days, produced hematuria in 31 to 100% of the patients. When MESNEX was administered together with these doses of ifosfamide, the incidence of hematuria was less than 7%.
Table 4. Percent of MESNEX Patients Developing Hematuria
(≥50 RBC/hpf or macrohematuria)Study
Conventional Uroprophylaxis (number of patients)
Standard MESNEX Intravenous Regimen (number of patients)
Uncontrolled Studies*
Study 1
16% (7/44)
-
Study 2
26% (11/43)
-
Study 3
18% (7/38)
0% (0/21)
Study 4
-
0% (0/32)
Controlled Studies†
Study 5
31% (14/46)
6% (3/46)
Study 6
100% (7/7)
0% (0/8)
*Ifosfamide dose 1.2 g/m2 d x 5
†Ifosfamide dose 2 g/m2 to 4 g/m2 d x 3 to 5
14.2 Oral MESNEX
Clinical studies comparing recommended intravenous and oral MESNEX dosing regimens demonstrated incidences of grade 3 to 4 hematuria of <5%. Study 7 was an open label, randomized, two-way crossover study comparing three intravenous doses with an initial intravenous dose followed by two oral doses of MESNEX in patients with cancer treated with ifosfamide at a dose of 1.2 g/m2 to 2.0 g/m2 for 3 to 5 days. Study 8 was a randomized, multicenter study in cancer patients receiving ifosfamide at 2.0 g/m2 for 5 days. In both studies, development of grade 3 or 4 hematuria was the primary efficacy endpoint. The percent of patients developing hematuria in each of these studies is presented in .
Table 5. Percent of MESNEX Patients Developing Grade 3 or 4 Hematuria
MESNEX Dosing Regimen
Study
Standard Intravenous Regimen
(number of patients)
Intravenous + Oral Regimen
(number of patients)
Study 7
0% (0/30)
3.6% (1/28)
Study 8
3.7% (1/27)
4.3% (1/23)
-
16 HOW SUPPLIED/STORAGE AND HANDLING
MESNEX (mesna) injection 100 mg/mL
- NDC: 0338-1307-05
- 1 g Multidose Vial, Box of 1 vial of 10 mL
- Store at 20°C to 25°C (68°F to 77°F), excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
-
17 PATIENT COUNSELING INFORMATION
- Advise the patient to discontinue MESNEX and seek immediate medical attention if any signs or symptoms of a hypersensitivity reaction, including systemic anaphylactic reactions occur [see Warnings and Precautions (5.1)].
- Advise the patient to take MESNEX at the exact time and in the exact amount as prescribed. Advise the patient to contact their healthcare provider if they vomit within 2 hours of taking oral MESNEX, or if they miss a dose of oral MESNEX [see Dosage and Administration (2.2)].
- MESNEX does not prevent hemorrhagic cystitis in all patients nor does it prevent or alleviate any of the other adverse reactions or toxicities associated with ifosfamide. Advise the patient to report to their healthcare provider if his/her urine has turned a pink or red color [see Dosage and Administration (2.3)].
- Advise the patient to drink 1 to 2 liters of fluid each day during MESNEX therapy [see Dosage and Administration (2.3)].
- Advise the patient that Stevens-Johnson syndrome, toxic epidermal necrolysis, and drug rash with eosinophilia and systemic symptoms and bullous and ulcerative skin and mucosal reactions have occurred with MESNEX. Advise the patient to report to their healthcare provider if signs and symptoms of these syndromes occur [see Warnings and Precautions (5.2)].
MESNEX (mesna) injection manufactured by:
MESNEX (mesna) tablets manufactured for:
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
- For Product Inquiry 1800 ANA DRUG (1-800-262-3784)
Made in Germany
NOVAPLUS is a registered trademark of Vizient, Inc.
Baxter, Mesnex, and Ifex are trademarks of Baxter International Inc.
HA-30-01-554
Revised May 2016
-
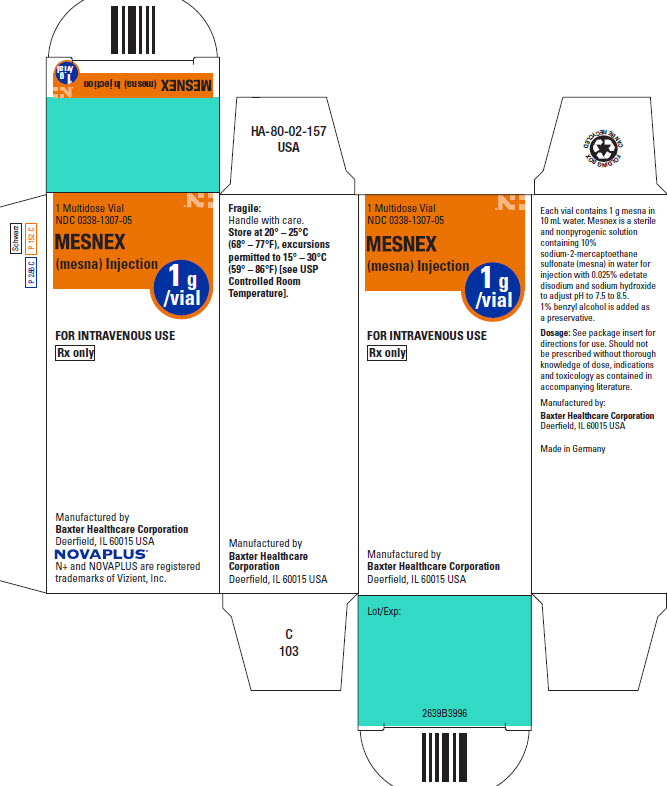
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
1 Multidose Vial
NDC: 0338-1307-10
MESNEX
(mesna) Injection1 g
/vialRx only
FOR INTRAVENOUS USE
NOVAPLUS Logo
N+ and NOVAPLUS are
registered trademarks
of Vizient, Inc.Each vial contains 1 gram of
mesna in 10 mL water.
1% benzyl alcohol is added
as a preservative.
See insert for dosing information.
Store at 20°C-25°C (68°-77°F),
excursions permitted to
15°-30°C (59°-86°F)
[see USP Controlled Room
Temperature].
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
USA HA-65-01-567 C 785Barcode
(01)10303381307100
Lot Number / Expires:MESNEX (mesna) Injection
1g
/vialSchwarz
P 286 C
P152 C1 Multidose Vial
NDC: 0338-1307-05MESNEX
(mesna) Injection1g
/vialRx only
Manufactured by:
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
NovaPlus Logo
N+ and NOVAPLUS are registered
trademarks of Vizient, Inc.FOR INTRAVENOUS USE
1 Multidose VialHA-80-02-157
USAFragile:
Handle with care.
Store at 20° – 25°C
(68° – 77°F), excursions
permitted to 15° – 30°C
(59° – 86°F) [see USP
Controlled Room
Temperature].Manufactured by:
Baxter Healthcare
Corporation
Deerfield, IL 60015 USAC
1031 Multidose Vial
NDC: 0338-1307-05MESNEX
(mesna) Injection1g
/vialFOR INTRAVENOUS USE
Rx OnlyManufactured by:
Baxter Healthcare Corporation
Deerfield, IL 60015 USALotT/Exp.:
2639B3996
Bar code
Folding Box Can Be Recycled Logo
Each vial contains 1 g mesna in
10 mL water. Mesnex is a sterile
and nonpyrogenic solution
containing 10%
sodium-2-mercaptoethane
sulfonate (mesna) in water
for injection with 0.025% edetate
disodium and sodium hydroxide
to adjust pH to 7.5 to 8.5.
1% benzyl alcohol is added as
a preservative.
Dosage: See package
insert for directions for
use. Should not be
prescribed without
thorough knowledge of
dose, indications and
toxicology as contained in
accompanying literature.Manufactured by:
Baxter Healthcare Corporation
Deerfield, IL 60015 USAMade in Germany
-
INGREDIENTS AND APPEARANCE
MESNEX
mesna injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0338-1307 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MESNA (UNII: NR7O1405Q9) (2-MERCAPTOETHANESULFONIC ACID - UNII:VHD28S0H7F) MESNA 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) 0.25 mg in 1 mL BENZYL ALCOHOL (UNII: LKG8494WBH) 1.04 mg in 1 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0338-1307-05 1 in 1 BOX 07/18/2018 1 NDC: 0338-1307-10 10 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019884 12/30/1988 Labeler - Baxter Healthcare Company (005083209) Establishment Name Address ID/FEI Business Operations Baxter Deutschland GmbH 312520353 API MANUFACTURE(0338-1307)
Trademark Results [MESNEX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MESNEX 85477540 4201334 Live/Registered |
BAXTER INTERNATIONAL INC. 2011-11-21 |
 MESNEX 73749527 1569152 Dead/Expired |
ASTA PHARMA AKTIENGESELLSCHAFT 1988-09-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.