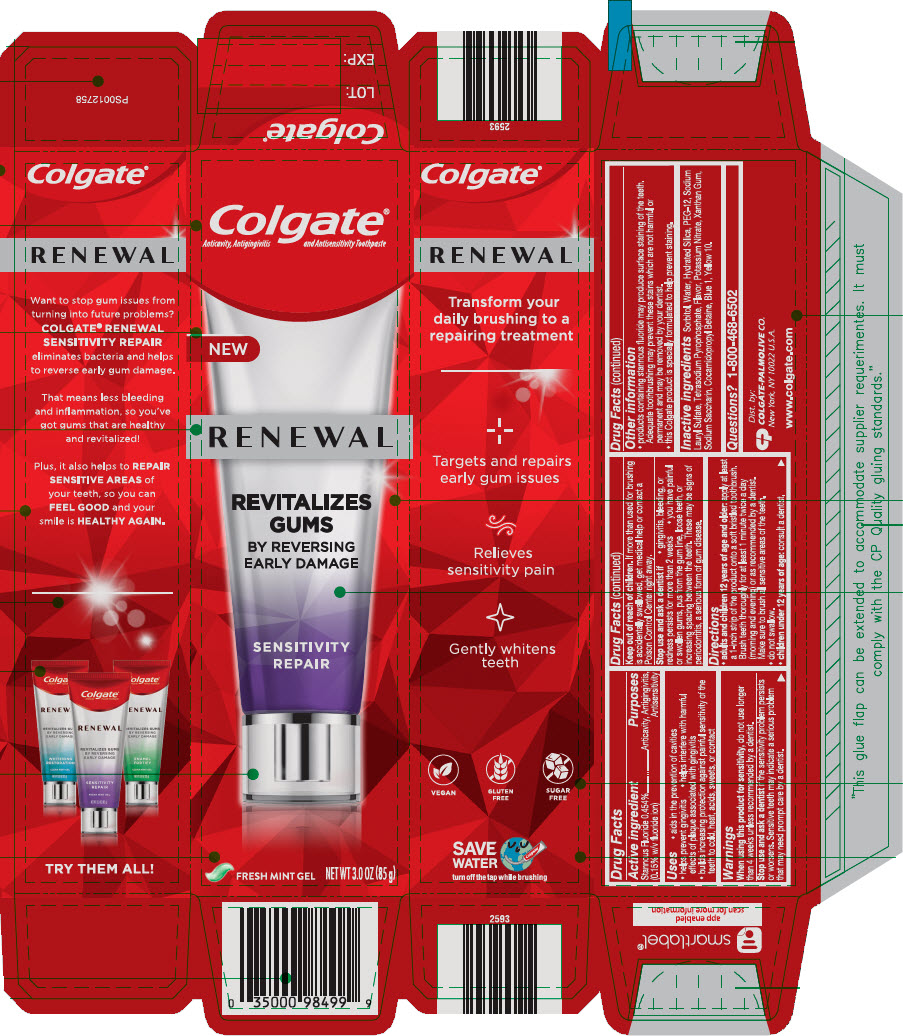
COLGATE RENEWAL SENSITIVITY REPAIR- stannous fluoride gel, dentifrice
Colgate-Palmolive Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Stannous Fluoride 0.454% (0.15% w/v fluoride ion)
Purposes
Anticavity, Antigingivitis, Antisensitivity
Uses
- aids in the prevention of cavities
- helps prevent gingivitis
- helps interfere with harmful effects of plaque associated with gingivitis
- builds increasing protection against painful sensitivity of the teeth to cold, heat, acids, sweets, or contact
Warnings
When using this product for sensitivity, do not use longer than 4 weeks unless recommended by a dentist.
Stop use and ask a dentist if the sensitivity problem persists or worsens. Sensitive teeth may indicate a serious problem that may need prompt care by a dentist.
Keep out of reach of children. If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Stop use and ask a dentist if
- gingivitis, bleeding, or redness persists for more than 2 weeks
- you have painful or swollen gums, pus from the gum line, loose teeth, or increasing spacing between the teeth. These may be signs of periodontitis, a serious form of gum disease.
Directions
-
adults and children 12 years of age and older: apply at least a 1-inch strip of the product onto a soft bristled toothbrush. Brush teeth thoroughly for at least 1 minute twice a day (morning and evening) or as recommended by a dentist. Make sure to brush all sensitive areas of the teeth.
- do not swallow.
-
children under 12 years of age: consult a dentist.
Other information
- products containing stannous fluoride may produce surface staining of the teeth. Adequate toothbrushing may prevent these stains which are not harmful or permanent and may be removed by your dentist.
- this Colgate product is specially formulated to help prevent staining.
Inactive ingredients
Sorbitol, Water, Hydrated Silica, PEG-12, Sodium Lauryl Sulfate, Tetrasodium Pyrophosphate, Flavor, Potassium Nitrate, Xanthan Gum, Sodium Saccharin, Cocamidopropyl Betaine, Blue 1, Yellow 10.
Questions?
1-800-468-6502
Dist. by:
COLGATE-PALMOLIVE CO.
New York, NY 10022 U.S.A.
PRINCIPAL DISPLAY PANEL - 85 g Tube Carton
Colgate®
Anticavity, Antigingivitis and Antisensitivity Toothpaste
GUM
RENEWAL
REVITALIZES
GUMS
BY REVERSING
EARLY DAMAGE
CLEANSING
3X
ACTION*
SENSITIVITY
NET WT 3.0 OZ (85 g)
GEL